Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release - PubMed (original) (raw)
Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release
Nadia De Mota et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Apelin, a recently isolated neuropeptide that is expressed in the supraoptic and the paraventricular nuclei, acts on specific receptors located on vasopressinergic neurons. The increased phasic pattern of these neurons facilitates sustained antidiuresis during dehydration or lactation. Here, we investigated whether apelin interacts with arginine vasopressin (AVP) to maintain body fluid homeostasis. We first characterized the predominant molecular forms of endogenous hypothalamic and plasma apelin as corresponding to apelin 13 and, to a lesser extent, to apelin 17. We then demonstrated that, in lactating rats, apelin was colocalized with AVP in supraoptic nucleus magnocellular neurons and given intracerebroventricularly inhibited the phasic electrical activity of AVP neurons. In lactating mice, intracerebroventricular administration of apelin 17 reduced plasma AVP levels and increased diuresis. Moreover, water deprivation, which increases systemic AVP release and causes depletion of hypothalamic AVP stores, decreased plasma apelin concentrations and induced hypothalamic accumulation of the peptide, indicating that AVP and apelin are conversely regulated to facilitate systemic AVP release and suppress diuresis. Opposite effects of AVP and apelin are likely to occur at the hypothalamic level through autocrine modulation of the phasic electrical activity of AVP neurons. Altogether, these data demonstrate that apelin acts as a potent diuretic neuropeptide counteracting AVP actions through inhibition of AVP neuron activity and AVP release. The coexistence of apelin and AVP in magnocellular neurons, their opposite biological effects, and regulation are likely to play a key role for maintaining body fluid homeostasis.
Figures
Fig. 1.
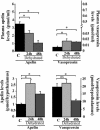
Distribution and characterization of apelin-related peptides in rat brain, pituitary gland, pineal gland, and plasma. (A) Apelin IR levels were quantified by RIA. Results are expressed in pmol/g of tissue or pmol/ml of plasma as the mean ± SEM of at least six independent experiments. (B and C) Apelin IR detected in gel permeation chromatography for fractions 20–40 of rat hypothalamic extracts (B) or fractions 20–30 of rat plasma (C). Results are expressed as the mean ± SEM of three independent experiments.
Fig. 2.
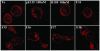
Effects of partially purified rat hypothalamic fractions on the internalization of the rat apelin receptor-EGFP. Confocal images of rat apelin receptor-EGFP CHO cells. Before ligand exposure (T0) or in presence of the inactive apelin fragment R10F (100 nM), the apelin receptor is confined to the plasma membrane. Internalization of the apelin receptor occurs in presence of pE13F (100 nM) or the rat hypothalamic fractions 35, 36, or 37.
Fig. 3.
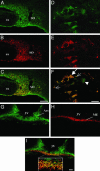
Confocal images of magnocellular neurons double-labeled with antisera against apelin and AVP in lactating rats. (A_–_C) Low-magnification views of the SON, showing the colocalization of apelin (green) (A) and AVP (red) (B). (D_–_F) High magnification views of the SON showing the colocalization (F) of apelin (D) with AVP (E) in cytoplasmic vesicles within the cell bodies (large arrow) and neuronal processes (small arrows). Some SON neurons (arrowhead) contain three distinct types of vesicles labeled for apelin, AVP, or both peptides (F). (G_–_I) Low-magnification views of the arcuate nucleus (Arc) and the internal zone of the median eminence (ME) that also contain apelin. (I Inset, internal zone) At higher magnification, many fibers were immunostained for apelin, AVP, or both peptides. In C, F, and I, yellow staining shows the colocalization of apelin and AVP. 3V, third ventricle; OX, optic chiasma. (Scale bar = 50 μmin A_–_C and G_–_I and 10 μmin D_–_F).
Fig. 4.
Electrical activity of AVP neurons in the SON of anesthetized lactating rats after central injection of K17F. The discontinuous display of firing rate (spikes per s) of a single AVP neuron shown here clearly illustrates the progressive installation and persistence of an inhibitory effect induced by injection of K17F (2 pmol) into the third ventricle.
Fig. 5.
Modification of plasma and hypothalamic apelin and AVP levels after water deprivation. Rats were deprived of water for 24 h (n = 10) or 48 h (n = 10). Plasma and hypothalamic apelin and AVP IR levels were measured by RIA. Mean ± SEM of six independent experiments carried out in duplicate.
Fig. 6.
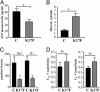
Effects of i.c.v. injection of K17F on systemic AVP release, diuresis, natriuresis, and kaliuresis in conscious lactating mice. (A) Systemic AVP release was estimated by measuring plasma AVP levels by RIA after the i.c.v. injection of saline or K17F (0.93 nmol = 2 μg) in lactating mice. (B_–_D) Diuresis (B), urinary concentrations of Na+ and K+ (C), and Na+ and K+ excretion rates (UNaV and UKV) (D) were measured for 3 h after the i.c.v. injection of saline or K17F (2 μg). Mean ± SEM, n = 5 in each group. *, P < 0.05 vs. control; ns, no significant difference vs. control animals.
Similar articles
- Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons.
Reaux-Le Goazigo A, Morinville A, Burlet A, Llorens-Cortes C, Beaudet A. Reaux-Le Goazigo A, et al. Endocrinology. 2004 Sep;145(9):4392-400. doi: 10.1210/en.2004-0384. Epub 2004 May 27. Endocrinology. 2004. PMID: 15166125 - Opposite potentiality of hypothalamic coexpressed neuropeptides, apelin and vasopressin in maintaining body-fluid homeostasis.
Llorens-Cortes C, Moos F. Llorens-Cortes C, et al. Prog Brain Res. 2008;170:559-70. doi: 10.1016/S0079-6123(08)00443-3. Prog Brain Res. 2008. PMID: 18655909 - [Apelin, a neuropeptide that counteracts vasopressin secretion].
Llorens-Cortès C, Beaudet A. Llorens-Cortès C, et al. Med Sci (Paris). 2005 Aug-Sep;21(8-9):741-6. doi: 10.1051/medsci/2005218-9741. Med Sci (Paris). 2005. PMID: 16115460 Review. French. - Role of the Vasopressin/Apelin Balance and Potential Use of Metabolically Stable Apelin Analogs in Water Metabolism Disorders.
Flahault A, Couvineau P, Alvear-Perez R, Iturrioz X, Llorens-Cortes C. Flahault A, et al. Front Endocrinol (Lausanne). 2017 May 31;8:120. doi: 10.3389/fendo.2017.00120. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28620355 Free PMC article. Review.
Cited by
- Apelin and energy metabolism.
Bertrand C, Valet P, Castan-Laurell I. Bertrand C, et al. Front Physiol. 2015 Apr 10;6:115. doi: 10.3389/fphys.2015.00115. eCollection 2015. Front Physiol. 2015. PMID: 25914650 Free PMC article. Review. - Advances in the study of ELABELA in renal physiological functions and related diseases.
Liu Y, Jiang M, Li Y, Chen P, Chen X. Liu Y, et al. Front Pharmacol. 2023 Oct 31;14:1276488. doi: 10.3389/fphar.2023.1276488. eCollection 2023. Front Pharmacol. 2023. PMID: 38026926 Free PMC article. Review. - Effect of volume expansion with hypertonic- and isotonic saline and isotonic glucose on sodium and water transport in the principal cells in the kidney.
Jensen JM, Mose FH, Bech JN, Nielsen S, Pedersen EB. Jensen JM, et al. BMC Nephrol. 2013 Sep 26;14:202. doi: 10.1186/1471-2369-14-202. BMC Nephrol. 2013. PMID: 24067081 Free PMC article. Clinical Trial. - Somato-dendritic vasopressin and oxytocin secretion in endocrine and autonomic regulation.
Brown CH, Ludwig M, Tasker JG, Stern JE. Brown CH, et al. J Neuroendocrinol. 2020 Jun;32(6):e12856. doi: 10.1111/jne.12856. Epub 2020 May 14. J Neuroendocrinol. 2020. PMID: 32406599 Free PMC article. Review. - Apelin, diabetes, and obesity.
Castan-Laurell I, Dray C, Attané C, Duparc T, Knauf C, Valet P. Castan-Laurell I, et al. Endocrine. 2011 Aug;40(1):1-9. doi: 10.1007/s12020-011-9507-9. Endocrine. 2011. PMID: 21725702 Review.
References
- Tatemoto, K., Hosoya, M., Habata, Y., Fujii, R., Kakegawa, T., Zou, M. X., Kawamata, Y., Fukusumi, S., Hinuma, S., Kitada, C., et al. (1998) Biochem. Biophys. Res. Commun. 251, 471–476. - PubMed
- O'Dowd, B. F., Heiber, M., Chan, A., Heng, H. H., Tsui, L. C., Kennedy, J. L., Shi, X., Petronis, A., George, S. R. & Nguyen, T. (1993) Gene 136, 355–360. - PubMed
- Habata, Y., Fujii, R., Hosoya, M., Fukusumi, S., Kawamata, Y., Hinuma, S., Kitada, C., Nishizawa, N., Murosaki, S., Kurokawa, T., et al. (1999) Biochim. Biophys. Acta. 13, 25–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous