Beta-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide - PubMed (original) (raw)
Beta-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide
Qinwen Wang et al. J Neurosci. 2004.
Abstract
The mechanisms underlying the inhibition of long-term potentiation (LTP) induction by amyloidbeta-peptide (Abeta) were investigated in the medial perforant path of the rat and mouse dentate gyrus in vitro. Evidence is presented in this study that the Abeta-mediated inhibition of LTP induction involves activation of microglia and production of reactive oxygen and nitrogen species. In control slices, Abeta strongly inhibited induction of NMDA receptor-dependent (NMDAR-dependent) LTP, although not induction of NMDAR-independent LTP or long-term depression (LTD). The inhibition of NMDAR-dependent LTP was prevented by minocycline, an agent that prevents activation of microglia. The involvement of inducible nitric oxide synthase (iNOS) was shown by the inability of Abeta to inhibit LTP induction in iNOS knock-out mice and also by the ability of two iNOS inhibitors, aminoguanidine and 1400W, to prevent the Abeta-mediated inhibition of LTP induction. The Abeta-mediated inhibition of LTP induction also was prevented by the superoxide scavenger superoxide dismutase applied together with catalase. Evidence for involvement of superoxide in the action of Abeta on LTP induction was shown by the ability of an inhibitor of NADPH oxidase to prevent the Abeta-mediated inhibition of LTP induction. The study thus provides evidence that the Abeta-mediated inhibition of LTP induction involves an inflammatory-type reaction in which activation of microglia results in the production of nitric oxide and superoxide and thence possibly peroxynitrite, a highly reactive oxidant.
Figures
Figure 1.
Aβ inhibits induction of NMDAR-dependent LTP, but not induction of NMDAR-independent LTP or LTD, in the rat hippocampus in vitro. A, NMDAR-dependent LTP induced by a single brief HFS in the medial perforant path of the dentate gyrus in control (filled circles) and in the presence of synthetic Aβ (500 n
m
), applied 40 min before HFS (open circles), reduced significantly from control. B, NMDAR-independent LTP induced by a single brief HFS in CA1 in control (filled circles) and in the presence of synthetic Aβ (1 μ
m
), applied 40 min before HFS (open circles), not reduced significantly from control. C, LTD induced by LFS (1 Hz, 15 min) in the dentate gyrus in control (filled circles) and in the presence of Aβ (500 n
m
), not reduced significantly from control. The traces above the graphs, labeled a-c in A and a, b in B and C, show field EPSPs at the times indicated. All data shown are the means ± SEM.
Figure 2.
Minocycline, an inhibitor of microglial activation, prevents the Aβ-mediated inhibition of LTP induction in the medial perforant path of the rat dentate gyrus in vitro. The graph shows the induction of LTP in the presence of minocycline (filled circles), not significantly different from control LTP, and in the presence of minocycline plus Aβ (open circles). Minocycline prevented the Aβ-mediated inhibition of LTP induction. All data shown are the means ± SEM. The traces a-c are the field EPSPs at the times indicated on the graph, with the top traces showing controls and the bottom traces showing the effect of Aβ.
Figure 3.
Aβ does not inhibit LTP in iNOS knock-out mice. A, Induction of LTP in the medial perforant path of the dentate gyrus of wild-type mice (filled circles) and in wild-type mice plus Aβ (open circles). Aβ inhibited LTP induction in the mouse dentate gyrus to an extent similar to that in the rat dentate gyrus. B, Induction of LTP in iNOS knock-out mice (filled circles), not significantly different from wild-type mice, and induction of LTP in the presence of Aβ in iNOS knock-out mice (open circles), not significantly different from control. All data shown are the means ± SEM. The traces a-c are the field EPSPs at the times indicated on the graph, with the top traces showing controls and the bottom traces showing the effect of Aβ.
Figure 4.
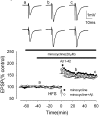
The Aβ-evoked inhibition of LTP induction is prevented by the iNOS inhibitors aminoguanidine and 1400W in the medial perforant path of the rat dentate gyrus. A, Induction of LTP in the presence of aminoguanidine (filled circles), not significantly reduced from control, and in the presence of aminoguanidine plus Aβ (open circles). Aminoguanidine prevented the Aβ-mediated inhibition of LTP induction. B, Induction of LTP in the presence of 1400W (filled circles), not significantly reduced from control, and in the presence of 1400W plus Aβ (open circles). 1400W prevented the Aβ-mediated inhibition of LTP induction. All data shown are the means ± SEM. The traces a-c are the field EPSPs at the times indicated on the graph, with the top traces showing controls and the bottom traces showing the effect of Aβ.
Figure 5.
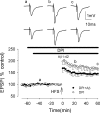
The Aβ-evoked inhibition of LTP induction is prevented by the superoxide scavenger SOD in rat slices. A, Induction of LTP in the presence of SOD (open circles), significantly reduced from control; in the presence of catalase (filled circles), not significantly reduced from control; and in the presence of SOD plus catalase, partially reduced from control. B, LTP induction in the presence of SOD plus catalase plus Aβ. The combination of SOD plus catalase prevented the Aβ-mediated inhibition of LTP induction. All data shown are the means ± SEM. The traces a-c are the field EPSPs at the times indicated on the graph, with the traces in A showing controls (catalase, SOD, and catalase plus SOD from top to bottom) and the traces in B showing catalase plus SOD plus Aβ.
Figure 6.
The Aβ-evoked inhibition of LTP induction is prevented by the NADPH oxidase inhibitor diphenyleneiodonium (DPI) in rat slices. The graph shows that DPI partially prevents the Aβ-mediated inhibition of LTP induction (filled circles). All data shown are the means ± SEM. The traces a-c are the field EPSPs at the times indicated on the graph, with the top traces showing controls and the bottom traces showing the effect of Aβ.
Similar articles
- NMDA receptor regulation by amyloid-beta does not account for its inhibition of LTP in rat hippocampus.
Raymond CR, Ireland DR, Abraham WC. Raymond CR, et al. Brain Res. 2003 Apr 11;968(2):263-72. doi: 10.1016/s0006-8993(03)02269-8. Brain Res. 2003. PMID: 12663096 - Enhancement of long-term depression by soluble amyloid β protein in rat hippocampus is mediated by metabotropic glutamate receptor and involves activation of p38MAPK, STEP and caspase-3.
Chen X, Lin R, Chang L, Xu S, Wei X, Zhang J, Wang C, Anwyl R, Wang Q. Chen X, et al. Neuroscience. 2013 Dec 3;253:435-43. doi: 10.1016/j.neuroscience.2013.08.054. Epub 2013 Sep 5. Neuroscience. 2013. PMID: 24012839 - Modulation of LTP induction by NMDA receptor activation and nitric oxide release.
Zorumski CF, Izumi Y. Zorumski CF, et al. Prog Brain Res. 1998;118:173-82. doi: 10.1016/s0079-6123(08)63207-0. Prog Brain Res. 1998. PMID: 9932441 Review. - Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo.
Weldon DT, Rogers SD, Ghilardi JR, Finke MP, Cleary JP, O'Hare E, Esler WP, Maggio JE, Mantyh PW. Weldon DT, et al. J Neurosci. 1998 Mar 15;18(6):2161-73. doi: 10.1523/JNEUROSCI.18-06-02161.1998. J Neurosci. 1998. PMID: 9482801 Free PMC article. Review.
Cited by
- How do Soluble Oligomers of Amyloid beta-protein Impair Hippocampal Synaptic Plasticity?
Li S, Shankar GM, Selkoe DJ. Li S, et al. Front Cell Neurosci. 2010 Mar 19;4:5. doi: 10.3389/fncel.2010.00005. eCollection 2010. Front Cell Neurosci. 2010. PMID: 20428510 Free PMC article. No abstract available. - NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits.
Weberpals M, Hermes M, Hermann S, Kummer MP, Terwel D, Semmler A, Berger M, Schäfers M, Heneka MT. Weberpals M, et al. J Neurosci. 2009 Nov 11;29(45):14177-84. doi: 10.1523/JNEUROSCI.3238-09.2009. J Neurosci. 2009. PMID: 19906966 Free PMC article. - RAGE inhibition in microglia prevents ischemia-dependent synaptic dysfunction in an amyloid-enriched environment.
Origlia N, Criscuolo C, Arancio O, Yan SS, Domenici L. Origlia N, et al. J Neurosci. 2014 Jun 25;34(26):8749-60. doi: 10.1523/JNEUROSCI.0141-14.2014. J Neurosci. 2014. PMID: 24966375 Free PMC article. - Fucoxanthin, a Marine Carotenoid, Attenuates _β_-Amyloid Oligomer-Induced Neurotoxicity Possibly via Regulating the PI3K/Akt and the ERK Pathways in SH-SY5Y Cells.
Lin J, Yu J, Zhao J, Zhang K, Zheng J, Wang J, Huang C, Zhang J, Yan X, Gerwick WH, Wang Q, Cui W, He S. Lin J, et al. Oxid Med Cell Longev. 2017;2017:6792543. doi: 10.1155/2017/6792543. Epub 2017 Aug 8. Oxid Med Cell Longev. 2017. PMID: 28928905 Free PMC article. - Role of Aβ in Alzheimer's-related synaptic dysfunction.
Zhang H, Jiang X, Ma L, Wei W, Li Z, Chang S, Wen J, Sun J, Li H. Zhang H, et al. Front Cell Dev Biol. 2022 Aug 26;10:964075. doi: 10.3389/fcell.2022.964075. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36092715 Free PMC article. Review.
References
- Akama KT, Van Eldik LJV (2000) β-Amyloid stimulation of inducible nitric oxide synthase in astrocytes is IL-1β- and TNF-α-dependent. J Biol Chem 275: 7918-7924. - PubMed
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, MacKenzie IR, McGeer PL, et al. (2000) Inflammation and Alzheimer's disease. Neurobiol Aging 21: 383-421. - PMC - PubMed
- Bianca VD, Dusi S, Bianchini E, Dal Pra I, Rossi F (1999) Beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer's disease. J Biol Chem 274: 15493-15499. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources