Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus - PubMed (original) (raw)
Comparative Study
Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus
Patrick C Y Woo et al. Clin Diagn Lab Immunol. 2004 Jul.
Abstract
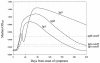
By using a recombinant severe acute respiratory syndrome coronavirus (SARS-CoV) nucleocapsid protein-based enzyme-linked immunosorbent assay (ELISA) and serum specimens serially collected (from day 0 to day 240 after symptom onset) from patients with pneumonia due to SARS-CoV, we analyzed the longitudinal profiles of immunoglobulin G (IgG), IgM, and IgA antibodies against the SARS-CoV nucleocapsid protein in patients with pneumonia due to SARS-CoV. For IgG, the median optical density at 450 nm (OD450) turned positive at day 17 and a biphasic response was observed. At day 240, all patients were still positive for anti-nucleocapsid protein IgG antibody. For IgM, the median OD450 turned positive at day 20.5, peaked at about day 80, and fell to below the baseline level at about day 180. At day 240, 36% of the patients were still positive for anti-nucleocapsid protein IgM antibody. For IgA, the median OD450 turned positive at day 17, peaked at about day 50, and fell to below the baseline level at about day 180. At day 240, 36% of the patients were still positive for anti-nucleocapsid protein IgA antibody. The time of seroconversion detected by the recombinant SARS-CoV nucleocapsid protein-based ELISA and that detected by indirect immunofluorescence assay were similar. The median times of seroconversion for IgG, IgM, and IgA detected by the indirect immunofluorescence assay were 17 days (17 days by ELISA), 16.5 days (20.5 days by ELISA), and 17.5 days (17 days by ELISA), respectively, after disease onset. One, four, and one of the six patients who died did not produce any IgG, IgM, and IgA antibodies against the nucleocapsid protein of SARS-CoV, respectively, although these antibodies were detected in all six patients by the indirect immunofluorescence assay. Further studies should be performed to see whether SARS-CoV nucleocapsid protein antibody positivity has any prognostic significance.
Figures
FIG. 1.
Longitudinal profile of IgG, IgM, and IgA antibodies to SARS-CoV nucleocapsid protein in patients with pneumonia due to SARS-CoV.
Similar articles
- Detection of specific antibodies to severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein for serodiagnosis of SARS coronavirus pneumonia.
Woo PC, Lau SK, Wong BH, Tsoi HW, Fung AM, Chan KH, Tam VK, Peiris JS, Yuen KY. Woo PC, et al. J Clin Microbiol. 2004 May;42(5):2306-9. doi: 10.1128/JCM.42.5.2306-2309.2004. J Clin Microbiol. 2004. PMID: 15131220 Free PMC article. - [A follow up study of total IgM, IgG, nucleoprotein and spike protein antibodies against severe acute respiratory syndrome (SARS) coronavirus in patients with SARS].
Yan HP, Tan YF, Zhuang H, Zhou YS, Zhao CH, Feng X, Jin RH, Wu H, Fu Y. Yan HP, et al. Zhonghua Nei Ke Za Zhi. 2006 Nov;45(11):896-9. Zhonghua Nei Ke Za Zhi. 2006. PMID: 17313874 Chinese. - Potential Usefulness of IgA for the Early Detection of SARS-CoV-2 Infection: Comparison With IgM.
Wang P. Wang P. Pol J Microbiol. 2024 Jun 20;73(2):123-130. doi: 10.33073/pjm-2024-019. eCollection 2024 Jun 1. Pol J Microbiol. 2024. PMID: 38905276 Free PMC article. Review. - Detection methods and dynamic characteristics of specific antibodies in patients with COVID-19: A review of the early literature.
Xu J, Chen J, Wen F, Liu K, Chen Y. Xu J, et al. Heliyon. 2024 Jan 24;10(3):e24580. doi: 10.1016/j.heliyon.2024.e24580. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38317938 Free PMC article. Review.
Cited by
- Clinical evaluation of SARS-CoV-2 point-of-care antibody tests.
Robosa RS, Sandaradura I, Dwyer DE, O'Sullivan MVN. Robosa RS, et al. Pathology. 2020 Dec;52(7):783-789. doi: 10.1016/j.pathol.2020.09.002. Epub 2020 Sep 22. Pathology. 2020. PMID: 33039095 Free PMC article. - Antibody responses against SARS coronavirus are correlated with disease outcome of infected individuals.
Zhang L, Zhang F, Yu W, He T, Yu J, Yi CE, Ba L, Li W, Farzan M, Chen Z, Yuen KY, Ho D. Zhang L, et al. J Med Virol. 2006 Jan;78(1):1-8. doi: 10.1002/jmv.20499. J Med Virol. 2006. PMID: 16299724 Free PMC article. - Evaluation of a recombinant nucleocapsid protein-based assay for anti-SARS-CoV IgG detection.
Chan PK, Liu EY, Leung DT, Cheung JL, Ma CH, Tam FC, Hui M, Tam JS, Lim PL. Chan PK, et al. J Med Virol. 2005 Feb;75(2):181-4. doi: 10.1002/jmv.20254. J Med Virol. 2005. PMID: 15602743 Free PMC article. - Duration of antibody responses after severe acute respiratory syndrome.
Wu LP, Wang NC, Chang YH, Tian XY, Na DY, Zhang LY, Zheng L, Lan T, Wang LF, Liang GD. Wu LP, et al. Emerg Infect Dis. 2007 Oct;13(10):1562-4. doi: 10.3201/eid1310.070576. Emerg Infect Dis. 2007. PMID: 18258008 Free PMC article.
References
- Guan, Y., B. J. Zheng, Y. Q. He, X. L. Liu, Z. X. Zhuang, C. L. Cheung, S. W. Luo, P. H. Li, L. J. Zhang, Y. J. Guan, K. M. Butt, K. L. Wong, K. W. Chan, W. Lim, K. F. Shortridge, K. Y. Yuen, J. S. Peiris, and L. L. Poon. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302:276-278. - PubMed
- Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, L. J. Anderson, and The SARS Working Group. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. - PubMed
- Li, G., X. Chen, and A. Xu. 2003. Profile of specific antibodies to the SARS-associated coronavirus. N. Engl. J. Med. 349:508-509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous