PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells - PubMed (original) (raw)
PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells
Yvette E Latchman et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Both positive and negative regulatory roles have been suggested for the B7 family member PD-L1(B7-H1). PD-L1 is expressed on antigen-presenting cells (APCs), activated T cells, and a variety of tissues, but the functional significance of PD-L1 on each cell type is not yet clear. To dissect the functions of PD-L1 in vivo, we generated PD-L1-deficient (PD-L1(-/-)) mice. CD4(+) and CD8(+) T cell responses were markedly enhanced in PD-L1(-/-) mice compared with wild-type mice in vitro and in vivo. PD-L1(-/-) dendritic cells stimulated greater wild-type CD4(+) T cell responses than wild-type dendritic cells, and PD-L1(-/-) CD4(+) T cells produced more cytokines than wild-type CD4(+) T cells in vitro, demonstrating an inhibitory role for PD-L1 on APCs and T cells. In vivo CD8(+) T cell responses also were significantly enhanced, indicating that PD-L1 has a role in limiting the expansion or survival of CD8(+) T cells. Studies using the myelin oligodendrocyte model of experimental autoimmune encephalomyelitis showed that PD-L1 on T cells and in host tissues limits responses of self-reactive CD4(+) T cells in vivo. PD-L1 deficiency converted the 129S4/SvJae strain from a resistant to experimental autoimmune encephalomyelitis-susceptible strain. Transfer of encephalitogenic T cells from wild-type mice into PD-L1(-/-) recipients led to exacerbated disease. Disease was even more severe in PD-L1(-/-) recipients of PD-L1(-/-) T cells. These results demonstrate that PD-L1 on T cells, APCs, and host tissue inhibits naïve and effector T cell responses and plays a critical role in T cell tolerance.
Figures
Fig. 1.
Generation of PD-L1–/– mice. (a) The structure of the PD-L1-targeting vector is shown (Top), and Neo replaced the signal exon and the IgV region (C57BL/6 construct). (Middle) The genomic organization of the PD-L1 gene (not to scale). Exons are open boxes. Homologous recombination of the PD-L1 gene is represented (Bottom). *, the position of the probe. (b) Southern blot analysis of PD-L1–/– mice. Wild-type DNA yields a 15.7-kb band and the targeted allele yields a 10.1-kb band. (c) Splenocytes were activated for 24 h with anti-CD3 (1 μg/ml) for T cells or LPS (10 μg/ml) for B cells. Flt-3-expanded DCs were isolated and matured overnight on plastic. Cells were stained with anti-PD-L1 FITC and relevant lineage-specific mAb-PE. Graphs are gated on CD4+, CD8+, CD19+, or CD11c+ cells as indicated. PD-L1+/+ mice (thick solid line), PD-L1–/– mice (dotted line), and isotype control (thin solid line).
Fig. 2.
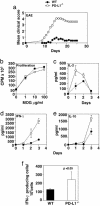
PD-L1 deficiency on the T cell and the APC enhanced IFN-γ production by T cells. To evaluate the role of PD-L1 on T cells, purified CD4+ T cells were stimulated with 1 μg/ml anti-CD3 plus 1 μg/ml anti-CD28 (a) and 10 μg/ml anti-CD3 plus 1 μg/ml anti-CD28 (b), and IFN-γ production was analyzed. (c) Purified CD8+ T cells were stimulated as in b, and IFN-γ production was analyzed. To examine the role of PD-L1 on the APC, C57BL/6 PD-L1+/+ or PD-L1–/– DC were compared as stimulators in a mixed lymphocyte reaction with BALB/c CD4+ T cells. DCs were expanded in vivo by Flt-3 ligand, isolated, matured overnight on plastic, irradiated, and cultured with BALB/c CD4+ T cells. IFN-γ (d), IL-2 (e), and IL-4 (f) were assayed by ELISA. These data are representative of three to six independent experiments.
Fig. 3.
Augmented CD8+ T cell clonal expansion and cytotoxic T lymphocyte responses in PD-L1–/– mice. (a) Mice were immunized with OVA in CFA, and 10 days later CD8+ T cells from LN cells were purified and restimulated with EL4-OVA or EL4 cells, and IFN-γ production assayed at days 1, 2, and 3. (b) LN cells were restimulated with EL4-OVA or EL4 cells and stained with CD8-FITC and Kb SIINFEKL tetramer-PE. Numbers in the upper right corner represent percent of CD8+ that was tetramer-positive. (c) Mice were immunized with OVA in CFA, and 10 days later splenocytes were restimulated with EL4-OVA cells for 5 days. Effector cells were recovered and plated with 51Cr-labeled SIINFEKL-pulsed EL4 at the indicated effector/target ratios. (d) Experiments were set up as in c, except CD8+ T cell numbers were normalized according to Kb SIINFEKL tetramer staining. These data are representative of four independent experiments.
Fig. 4.
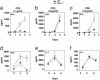
Increased susceptibility to EAE in PD-L1–/– mice. (a) 129Sv PD-L1–/– (○) and PD-L1+/+ (•) mice were immunized with MOG33–55 and mice were scored daily. (b_–_f) To assess MOG-specific responses, mice were immunized with MOG33–55, and 10 days later draining LN cells were harvested and restimulated with MOG33–55. (b) Proliferation was measured at day 2, and IL-2 (c), IFN-γ (d), and IL-10 (e) were assayed from days 0 to 4 by ELISA. To determine the number of antigen-specific IFN-γ producing cells, draining LN cells were restimulated with MOG33–55 for 24 h, and (f) enzyme-linked immunospot assays were performed. These data are representative of three to four independent experiments.
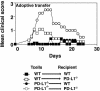
Fig. 5.
Effector phase of EAE is exacerbated in the absence of PD-L1. Mice were immunized with MOG33–55. LN cells were harvested 10 days later, restimulated with MOG33–55 for 4 days, and transferred into PD-L1+/+ or PD-L1–/– recipients as indicated. These data are representative of four independent experiments.
Similar articles
- PD-L1/PD-1 signal deficiency promotes allogeneic immune responses and accelerates heart allograft rejection.
Wang W, Carper K, Malone F, Latchman Y, Perkins J, Fu Y, Reyes J, Li W. Wang W, et al. Transplantation. 2008 Sep 27;86(6):836-44. doi: 10.1097/TP.0b013e3181861932. Transplantation. 2008. PMID: 18813109 - Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis.
Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, Wiendl H. Schreiner B, et al. J Neuroimmunol. 2004 Oct;155(1-2):172-82. doi: 10.1016/j.jneuroim.2004.06.013. J Neuroimmunol. 2004. PMID: 15342209 - Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis.
Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Rodig N, et al. Eur J Immunol. 2003 Nov;33(11):3117-26. doi: 10.1002/eji.200324270. Eur J Immunol. 2003. PMID: 14579280 - CD40-activated B cells as antigen-presenting cells: the final sprint toward clinical application.
Wennhold K, Shimabukuro-Vornhagen A, Theurich S, von Bergwelt-Baildon M. Wennhold K, et al. Expert Rev Vaccines. 2013 Jun;12(6):631-7. doi: 10.1586/erv.13.39. Expert Rev Vaccines. 2013. PMID: 23750793 Review. - Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade.
Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Freeman GJ, et al. J Exp Med. 2006 Oct 2;203(10):2223-7. doi: 10.1084/jem.20061800. Epub 2006 Sep 25. J Exp Med. 2006. PMID: 17000870 Free PMC article. Review.
Cited by
- Interleukin-27 and Its Diverse Effects on Bacterial Infections.
Morita Y, Masters EA, Schwarz EM, Muthukrishnan G. Morita Y, et al. Front Immunol. 2021 May 17;12:678515. doi: 10.3389/fimmu.2021.678515. eCollection 2021. Front Immunol. 2021. PMID: 34079555 Free PMC article. Review. - PD-1 restraint of regulatory T cell suppressive activity is critical for immune tolerance.
Tan CL, Kuchroo JR, Sage PT, Liang D, Francisco LM, Buck J, Thaker YR, Zhang Q, McArdel SL, Juneja VR, Lee SJ, Lovitch SB, Lian C, Murphy GF, Blazar BR, Vignali DAA, Freeman GJ, Sharpe AH. Tan CL, et al. J Exp Med. 2021 Jan 4;218(1):e20182232. doi: 10.1084/jem.20182232. J Exp Med. 2021. PMID: 33045061 Free PMC article. - IL-10 producing B cells partially restore E2-mediated protection against EAE in PD-L1 deficient mice.
Zhang J, Benedek G, Bodhankar S, Lapato A, Vandenbark AA, Offner H. Zhang J, et al. J Neuroimmunol. 2015 Aug 15;285:129-36. doi: 10.1016/j.jneuroim.2015.06.002. Epub 2015 Jun 11. J Neuroimmunol. 2015. PMID: 26198929 Free PMC article. - Immune checkpoints in central nervous system autoimmunity.
Joller N, Peters A, Anderson AC, Kuchroo VK. Joller N, et al. Immunol Rev. 2012 Jul;248(1):122-39. doi: 10.1111/j.1600-065X.2012.01136.x. Immunol Rev. 2012. PMID: 22725958 Free PMC article. Review. - Synergistic upregulation of PD‑L1 in tumor cells and CD39 in tumor‑infiltrating CD8+ T cells leads to poor prognosis in patients with hepatocellular carcinoma.
Kang X, Zhao S, Lin S, Li J, Wang S. Kang X, et al. Oncol Lett. 2024 Jun 12;28(2):368. doi: 10.3892/ol.2024.14501. eCollection 2024 Aug. Oncol Lett. 2024. PMID: 38933811 Free PMC article.
References
- Agata, Y., Kawasaki, A., Nishimura, H., Ishida, Y., Tsubata, T., Yagita, H. & Honjo, T. (1996) Int. Immunol. 8, 765–772. - PubMed
- Ishida, M., Iwai, Y., Tanaka, Y., Okazaki, T., Freeman, G., Minato, N. & Honjo, T. (2002) Immunol. Lett. 84, 57–62. - PubMed
- Nishimura, H., Nose, M., Hiai, H., Minato, N. & Honjo, T. (1999) Immunity 11, 141–151. - PubMed
- Nishimura, H., Okazaki, T., Tanaka, Y., Nakatani, K., Hara, M., Matsumori, A., Sasayama, S., Mizoguchi, A., Hiai, H., Minato, N. & Honjo, T. (2001) Science 291, 319–322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous