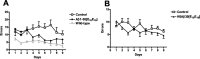An attenuated immune response is sufficient to enhance cognition in an Alzheimer's disease mouse model immunized with amyloid-beta derivatives - PubMed (original) (raw)
Comparative Study
An attenuated immune response is sufficient to enhance cognition in an Alzheimer's disease mouse model immunized with amyloid-beta derivatives
Einar M Sigurdsson et al. J Neurosci. 2004.
Abstract
Immunization with amyloid-beta (Abeta) 1-42 has been shown to reduce amyloid burden and improve cognition in Alzheimer's disease (AD) model mice. In a human trial, possible cognitive benefit was found but in association with significant toxicity in a minority of patients. We proposed that immunization with nonfibrillogenic Abeta derivatives is much less likely to produce toxicity and have previously shown that one such derivative (K6Abeta1-30) can reduce amyloid burden in mice to a similar extent as Abeta1-42. Here, we immunized AD model mice (Tg2576) with Abeta1-30[E18E19] or with K6Abeta1-30[E18E19]. These peptides were designed to be nontoxic and to produce less T-cell response, which has been linked to toxicity. K6Abeta1-30[E18E19] induced primarily an IgM response, whereas Abeta1-30[E18E19] induced an IgG titer that was lower than previously seen with K6Abeta1-30 or Abeta1-42. However, both treated animal groups performed better than Tg controls in the radial arm maze. Amyloid burden was similar in Abeta1-30[E18E19]-vaccinated mice and their Tg controls, whereas the number of medium and small sized plaques was reduced (29-34%) in K6Abeta1-30[E18E19]-immunized mice compared with Tg controls. Amyloid burden in these mice correlated inversely with plasma IgM levels. The cognitive benefit and amyloid reduction in the K6Abeta1-30[E18E19]-vaccinated mice are likely to be related to peripheral clearance of Abeta, because IgM does not cross the blood-brain barrier because of its large size. Our results indicate that these nontoxic Abeta derivatives produce an attenuated antibody response, which is less likely to be associated with negative side effects while having cognitive benefits.
Figures
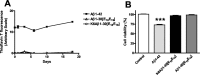
Figure 1.
A, Thioflavin T fluorometric assay. Fibril formation of Aβ1-42, Aβ1-30[E18E19], and K6Aβ1-30[E18E19] was measured in vitro in triplicates (37°C; 0-18 d). The Aβ derivatives were not fibrillogenic compared with Aβ1-42, which readily formed fibrils. This particular lot of Aβ1-42 already formed fibrils at _t_=0, and the amount of fibrils did not increase substantially over time. B, MTT assay. Aβ1-42 was toxic to human neuroblastoma cells (SK-N-SH) in culture, whereas the Aβ derivatives had no effect compared with the control group. ***p < 0.001, compared with control group (one-way ANOVA; Neuman-Keuls post hoc test).
Figure 2.
A, Group differences were observed in the radial arm maze (two-way ANOVA, repeated measures; treatment, p < 0.0001; days, p = 0.03). Vehicle-treated (n = 8) transgenic mice performed significantly worse in the maze compared with Aβ1-30[E18E19]-treated transgenic mice (p = 0.02; n = 6) and their wild-type littermates (p < 0.001; n = 17). B, K6Aβ1-30[E18E19]-treated transgenic mice (n = 13) had significantly fewer errors in the radial arm maze compared with their vehicle-treated (n = 19) controls (two-way ANOVA, repeated measures; treatment, p < 0.05; days, p = 0.02).
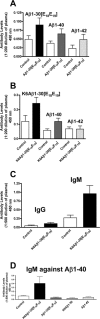
Figure 3.
A, Immunization with Aβ1-30[E18E19] resulted in a modest increase in IgG recognizing the antigen (black), Aβ1-40 (dark gray), and Aβ1-42 (light gray) on ELISA plates as detected in plasma samples diluted 1:200 obtained at the end of the study (n = 6-8 per group). Controls are plasma samples from mice injected with adjuvant and peptide vehicle incubated on the same peptide-coated ELISA plates as plasma from Aβ1-30[E18E19]. The peptides used for coating the plates are listed above the respective bars. B, Likewise, immunization with K6Aβ1-30[E18E19] elicited a modest IgG response against the antigens Aβ1-40 and Aβ1-42 at the same plasma dilution (n = 14-18 per group). The controls are as described in A, and the peptides used for coating the ELISA plates are listed above the bars. C, K6Aβ1-30[E18E19] induced a substantially more pronounced IgM response against itself compared with IgG response as detected in plasma at 1:500 dilution. The controls are as described in A. D, The IgM antibodies generated after K6Aβ1-30[E18E19] immunization cross-reacted with Aβ1-40 (n = 12). Plasma from mice immunized with other Aβ derivatives, Aβ1-42 (n = 6-9 per group) or control mice that received adjuvant with peptide vehicle (n = 16), had minimal IgM reactivity toward Aβ1-40.
Figure 4.
Immunization with K6Aβ1-30[E18E19] preferentially reduced small (A) (34% reduction; p = 0.02) and medium-sized (B) (29% reduction; p = 0.04) plaques. C, Large plaques were not significantly affected (n = 18 per group). D, E, Low amyloid plaque burden correlated with high IgM levels against the immunogen (D) (p < 0.05) and Aβ1-42 (E) (p = 0.05). F, A trend for correlation was seen for IgM recognizing Aβ1-40.
Similar articles
- Antibody response and plasma Abeta1-40 levels in young Microcebus murinus primates immunized with Abeta1-42 and its derivatives.
Trouche SG, Asuni A, Rouland S, Wisniewski T, Frangione B, Verdier JM, Sigurdsson EM, Mestre-Francés N. Trouche SG, et al. Vaccine. 2009 Feb 11;27(7):957-64. doi: 10.1016/j.vaccine.2008.12.012. Epub 2008 Dec 27. Vaccine. 2009. PMID: 19114076 Free PMC article. - Short amyloid-beta (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer's disease mouse model in the absence of an Abeta-specific cellular immune response.
Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, Lemere CA. Maier M, et al. J Neurosci. 2006 May 3;26(18):4717-28. doi: 10.1523/JNEUROSCI.0381-06.2006. J Neurosci. 2006. PMID: 16672644 Free PMC article. - Lack of P-glycoprotein Results in Impairment of Removal of Beta-Amyloid and Increased Intraparenchymal Cerebral Amyloid Angiopathy after Active Immunization in a Transgenic Mouse Model of Alzheimer's Disease.
Bruckmann S, Brenn A, Grube M, Niedrig K, Holtfreter S, von Bohlen und Halbach O, Groschup M, Keller M, Vogelgesang S. Bruckmann S, et al. Curr Alzheimer Res. 2017;14(6):656-667. doi: 10.2174/1567205013666161201201227. Curr Alzheimer Res. 2017. PMID: 27915995 - Novel Abeta immunogens: is shorter better?
Lemere CA, Maier M, Peng Y, Jiang L, Seabrook TJ. Lemere CA, et al. Curr Alzheimer Res. 2007 Sep;4(4):427-36. doi: 10.2174/156720507781788800. Curr Alzheimer Res. 2007. PMID: 17908047 Review. - Developing novel immunogens for a safe and effective Alzheimer's disease vaccine.
Lemere CA. Lemere CA. Prog Brain Res. 2009;175:83-93. doi: 10.1016/S0079-6123(09)17506-4. Prog Brain Res. 2009. PMID: 19660650 Free PMC article. Review.
Cited by
- Treatment strategies targeting amyloid β-protein.
Schenk D, Basi GS, Pangalos MN. Schenk D, et al. Cold Spring Harb Perspect Med. 2012 Sep 1;2(9):a006387. doi: 10.1101/cshperspect.a006387. Cold Spring Harb Perspect Med. 2012. PMID: 22951439 Free PMC article. Review. - Amyloid-beta immunotherapy for Alzheimer's disease.
Fu HJ, Liu B, Frost JL, Lemere CA. Fu HJ, et al. CNS Neurol Disord Drug Targets. 2010 Apr;9(2):197-206. doi: 10.2174/187152710791012017. CNS Neurol Disord Drug Targets. 2010. PMID: 20205640 Free PMC article. Review. - Amyloid-beta peptide remnants in AN-1792-immunized Alzheimer's disease patients: a biochemical analysis.
Patton RL, Kalback WM, Esh CL, Kokjohn TA, Van Vickle GD, Luehrs DC, Kuo YM, Lopez J, Brune D, Ferrer I, Masliah E, Newel AJ, Beach TG, Castaño EM, Roher AE. Patton RL, et al. Am J Pathol. 2006 Sep;169(3):1048-63. doi: 10.2353/ajpath.2006.060269. Am J Pathol. 2006. PMID: 16936277 Free PMC article. - Plasma protein profiling of Mild Cognitive Impairment and Alzheimer's disease using iTRAQ quantitative proteomics.
Song F, Poljak A, Kochan NA, Raftery M, Brodaty H, Smythe GA, Sachdev PS. Song F, et al. Proteome Sci. 2014 Jan 17;12(1):5. doi: 10.1186/1477-5956-12-5. Proteome Sci. 2014. PMID: 24433274 Free PMC article. - DNA prime-protein boost increased the titer, avidity and persistence of anti-Abeta antibodies in wild-type mice.
Davtyan H, Mkrtichyan M, Movsesyan N, Petrushina I, Mamikonyan G, Cribbs DH, Agadjanyan MG, Ghochikyan A. Davtyan H, et al. Gene Ther. 2010 Feb;17(2):261-71. doi: 10.1038/gt.2009.140. Epub 2009 Oct 29. Gene Ther. 2010. PMID: 19865176 Free PMC article.
References
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T (2000) Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6: 916-919. - PubMed
- Baumgarth N (2000) A two-phase model of B-cell activation. Immunol Rev 176: 171-180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG20197/AG/NIA NIH HHS/United States
- R37 AG005891/AG/NIA NIH HHS/United States
- R01 AR002594/AR/NIAMS NIH HHS/United States
- R01 AG020245/AG/NIA NIH HHS/United States
- R01 AG020197/AG/NIA NIH HHS/United States
- AG05891/AG/NIA NIH HHS/United States
- AR02594/AR/NIAMS NIH HHS/United States
- AG20245/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous