Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas - PubMed (original) (raw)
Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas
Koichi Shimizu et al. J Clin Invest. 2004 Jul.
Erratum in
- J Clin Invest. 2004 Sep;114(5):739
Abstract
Abdominal aortic aneurysms (AAAs) cause death due to complications related to expansion and rupture. The underlying mechanisms that drive AAA development remain largely unknown. We recently described evidence for a shift toward T helper type 2 (Th2) cell responses in human AAAs compared with stenotic atheromas. To evaluate putative pathways in AAA formation, we induced Th1- or Th2-predominant cytokine environments in an inflammatory aortic lesion using murine aortic transplantation into WT hosts or those lacking the receptors for the hallmark Th1 cytokine IFN-gamma, respectively. Allografts in WT recipients developed intimal hyperplasia, whereas allografts in IFN-gamma receptor-deficient (GRKO) hosts developed severe AAA formation associated with markedly increased levels of MMP-9 and MMP-12. Allografts in GRKO recipients treated with anti-IL-4 antibody to block the characteristic IL-4 Th2 cytokine or allografts in GRKO hosts also congenitally deficient in IL-4 did not develop AAA and likewise exhibited attenuated collagenolytic and elastolytic activities. These observations demonstrate an important dichotomy between cellular immune responses that induce IFN-gamma- or IL-4-dominated cytokine environments. The findings establish important regulatory roles for a Th1/Th2 cytokine balance in modulating matrix remodeling and have important implications for the pathophysiology of AAAs and arteriosclerosis.
Figures
Figure 1
Aortic allografts from WT or GRKO recipients were harvested 1 week after transplantation and were analyzed for cytokine expression by RNase protection assay (RPA) (A and B) and Western blot for IL-4 (C). (A) Gel image of RPA. (B) Histogram of the optical densities from RPA data normalized to GAPDH and averaged for 6 or 7 grafts. (C) Protein extracts (25 μg/lane) of the aortic grafts of WT or GRKO recipients analyzed by Western blot for IL-4.
Figure 2
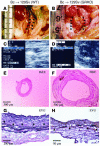
Aortic allografts from WT (A, C, E, and G) or GRKO (B, D, F, and H) recipients 12 weeks after transplantation. (A and B) Representative gross appearance of aortas. (C and D) Echoaortograms. (E and F) Histologic appearance of transverse sections. (G and H) Elastic van Gieson staining (EVG). Analysis of 9–10 aortic transplants from different donors yielded similar results. Control (WT) experiments, shown in panels A, C, E, and G, did not reveal measurable aneurysm formation or elastic tissue degradation. Arrowheads indicate elastic lamellae.
Figure 3
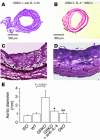
Aortic allografts from GRKO recipients treated with anti–IL-4 antibody (A and C) or from DKO recipients (B and D) harvested at 12 weeks after transplantation. (A and B) Representative histologic appearance of transverse sections. (C and D) Elastic van Gieson staining. (E) Analysis of aortic diameter from the isograft (Iso) (n = 5), WT recipient allografts (n = 9), GRKO recipient allografts (n = 10), allografts in GRKO recipients with anti–IL-4 antibody (11B11) treatment (n = 3), and DKO (n = 6) recipient allografts 12 weeks after transplantation. *P < 0.01 vs. GRKO; **P < 0.0001 vs. GRKO.
Figure 4
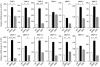
Metalloproteinase expression during AAA development. The MMP mRNA levels of aortic allografts from WT (white bars), GRKO (black bars), or DKO (gray bars) recipients (n = 6, each). Total RNA was extracted from the aortic allografts and first-strand cDNA generated by reverse transcription was subjected to real-time quantitative PCR with the LightCycler. Data represent the mean ± SEM of six determinations of the percentage of mRNA copies relative to untreated growing cells. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 5
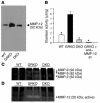
Western blot for MMP-12, elastase colorimetric assay, and gelatin and casein zymography. (A) Representative gel image of Western blot analysis. Protein extracts (20 μg/lane) of the aortic grafts of WT (n = 6), GRKO (n = 6), or DKO (n = 6) recipients analyzed by Western blot for MMP-12. The gel images represent qualitatively similar results. (B) The elastase colorimetric assay shows significantly greater elastase activity in the proteins extracted from allografts in GRKO hosts (n = 6) compared with proteins extracted from allografts in WT (n = 6) or DKO (n = 6) hosts. After anti–MMP-12 immunoprecipitation (IP) (n = 6), the proteins from allografts from GRKO recipients had significantly reduced elastase activity, which indicates that the majority of elastolytic activity in those allografts derives from MMP-12. Bar shows mean ± SEM; *P < 0.0001. (C and D) Representative gel images of gelatin zymogram (C) and casein zymogram (D). Protein extracts (20 μg/lane) of the aortic grafts of WT (n = 6), GRKO (n = 6), or DKO (n = 6) recipients analyzed by (C) gelatin- or (D) casein-zymogram for MMPs. The gel images represent qualitatively similar results. We could detect only 92 kDa and 72 kDa active bands from GRKO recipient allografts in the gelatin zymogram (C) and only 20 kDa active band from GRKO recipient allografts in the casein zymogram (D).
Figure 6
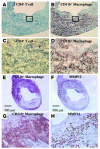
Representative immunohistochemistry of CD4+ (A and C) and CD11b+ (B and D) cells in allografts from GRKO recipients harvested 4 weeks after transplantation (n = 6). Boxed area in A or B (original magnification, ×100) is enlarged in C or D (original magnification, ×400), respectively. The majority of the graft infiltrating cells, especially adjacent to degraded elastic lamellae, consisted of CD11b+ macrophages. Expression of MMP-12 (F and H) colocalized with CD11b+ cells (E and G) in allografts from GRKO recipients harvested 4 weeks after transplantation (n = 6).
Figure 7
Conventional RT-PCR (A) and real-time PCR (B) for MMP-12 from WT bone marrow×derived macrophages incubated 18 hours with IL-4 (10 ng/ml), IFN-γ (500 U/ml), and/or anti×IL-4 antibody (5 μg/ml, 11B11). IL-4 augmented and IFN-γ inhibited MMP-12 mRNA expression of the WT macrophages. In addition, 11B11 inhibited IL-4×induced augmentation of MMP-12 mRNA expression by the WT macrophages. Bar shows mean ± SEM (n = 6, each). *P < 0.0001.
Comment in
- Adaptive cellular immunity in aortic aneurysms: cause, consequence, or context?
Curci JA, Thompson RW. Curci JA, et al. J Clin Invest. 2004 Jul;114(2):168-71. doi: 10.1172/JCI22309. J Clin Invest. 2004. PMID: 15254583 Free PMC article.
Similar articles
- Inflammation and cellular immune responses in abdominal aortic aneurysms.
Shimizu K, Mitchell RN, Libby P. Shimizu K, et al. Arterioscler Thromb Vasc Biol. 2006 May;26(5):987-94. doi: 10.1161/01.ATV.0000214999.12921.4f. Epub 2006 Feb 23. Arterioscler Thromb Vasc Biol. 2006. PMID: 16497993 Review. - Local cytokine environments drive aneurysm formation in allografted aortas.
Shimizu K, Libby P, Mitchell RN. Shimizu K, et al. Trends Cardiovasc Med. 2005 May;15(4):142-8. doi: 10.1016/j.tcm.2005.05.003. Trends Cardiovasc Med. 2005. PMID: 16099378 Review. - Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model.
Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Xiong W, et al. J Immunol. 2004 Feb 15;172(4):2607-12. doi: 10.4049/jimmunol.172.4.2607. J Immunol. 2004. PMID: 14764734 - Predominance of type 1 CD4+ T cells in human abdominal aortic aneurysm.
Galle C, Schandené L, Stordeur P, Peignois Y, Ferreira J, Wautrecht JC, Dereume JP, Goldman M. Galle C, et al. Clin Exp Immunol. 2005 Dec;142(3):519-27. doi: 10.1111/j.1365-2249.2005.02938.x. Clin Exp Immunol. 2005. PMID: 16297165 Free PMC article. - MHC-matched corneal allograft rejection in an IFN-gamma/IL-17-independent manner in C57BL/6 mice.
Yamada J, Hamuro J, Fukushima A, Ohteki T, Terai K, Iwakura Y, Yagita H, Kinoshita S. Yamada J, et al. Invest Ophthalmol Vis Sci. 2009 May;50(5):2139-46. doi: 10.1167/iovs.08-2993. Epub 2009 Jan 10. Invest Ophthalmol Vis Sci. 2009. PMID: 19136699
Cited by
- SOX6 expression and aneurysms of the thoracic and abdominal aorta.
Carmona-Berrio D, Adarve-Rengifo I, Marshall AG, Vue Z, Hall DD, Miller-Fleming TW, Actkins KV, Beasley HK, Almonacid PM, Barturen-Larrea P, Wells QS, Lopez MG, Garza-Lopez E, Dai DF, Shao J, Neikirk K, Billings FT 4th, Curci JA, Cox NJ, Gama V, Hinton A Jr, Gomez JA. Carmona-Berrio D, et al. iScience. 2024 Jul 24;27(9):110436. doi: 10.1016/j.isci.2024.110436. eCollection 2024 Sep 20. iScience. 2024. PMID: 39262802 Free PMC article. - A porcine model of thoracic aortic aneurysms created with a retrievable drug infusion stent graft mirrors human aneurysm pathophysiology.
Kenawy DM, Stafford JF, Amari F, Campbell D, Abdel-Rasoul M, Leight J, Chun Y, Tillman BW. Kenawy DM, et al. JVS Vasc Sci. 2024 Jul 2;5:100212. doi: 10.1016/j.jvssci.2024.100212. eCollection 2024. JVS Vasc Sci. 2024. PMID: 39188992 Free PMC article. - Mmp12 Is Translationally Regulated in Macrophages during the Course of Inflammation.
Kuntschar S, Cardamone G, Klann K, Bauer R, Meyer SP, Raue R, Rappl P, Münch C, Brüne B, Schmid T. Kuntschar S, et al. Int J Mol Sci. 2023 Nov 30;24(23):16981. doi: 10.3390/ijms242316981. Int J Mol Sci. 2023. PMID: 38069304 Free PMC article. - T cells in abdominal aortic aneurysm: immunomodulation and clinical application.
Gong W, Tian Y, Li L. Gong W, et al. Front Immunol. 2023 Aug 18;14:1240132. doi: 10.3389/fimmu.2023.1240132. eCollection 2023. Front Immunol. 2023. PMID: 37662948 Free PMC article. Review. - The immunomodulatory role of matrix metalloproteinases in colitis-associated cancer.
He L, Kang Q, Chan KI, Zhang Y, Zhong Z, Tan W. He L, et al. Front Immunol. 2023 Jan 19;13:1093990. doi: 10.3389/fimmu.2022.1093990. eCollection 2022. Front Immunol. 2023. PMID: 36776395 Free PMC article. Review.
References
- Hallett JW., Jr Management of abdominal aortic aneurysms. Mayo Clin. Proc. 2000;75:395–399. - PubMed
- Glagov S. Morphology of collagen and elastin fibers in atherosclerotic lesions. Adv. Exp. Med. Biol. 1977;82:767–773. - PubMed
- Campa JS, Greenhalgh RM, Powell JT. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis. 1987;65:13–21. - PubMed
- Newman KM, et al. Matrix metalloproteinases in abdominal aortic aneurysm: characterization, purification, and their possible sources. Connect. Tissue Res. 1994;30:265–276. - PubMed
- Thompson RW, Parks WC. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann. N. Y. Acad. Sci. 1996;800:157–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM067049/GM/NIGMS NIH HHS/United States
- HL-67249/HL/NHLBI NIH HHS/United States
- R01 HL-43364/HL/NHLBI NIH HHS/United States
- R01 HL067283/HL/NHLBI NIH HHS/United States
- GM-67049/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous