Pathogenesis of malaria and clinically similar conditions - PubMed (original) (raw)
Review
Pathogenesis of malaria and clinically similar conditions
Ian A Clark et al. Clin Microbiol Rev. 2004 Jul.
Abstract
There is now wide acceptance of the concept that the similarity between many acute infectious diseases, be they viral, bacterial, or parasitic in origin, is caused by the overproduction of inflammatory cytokines initiated when the organism interacts with the innate immune system. This is also true of certain noninfectious states, such as the tissue injury syndromes. This review discusses the historical origins of these ideas, which began with tumor necrosis factor (TNF) and spread from their origins in malaria research to other fields. As well the more established proinflammatory mediators, such as TNF, interleukin-1, and lymphotoxin, the roles of nitric oxide and carbon monoxide, which are chiefly inhibitory, are discussed. The established and potential roles of two more recently recognized contributors, overactivity of the enzyme poly(ADP-ribose) polymerase 1 (PARP-1) and the escape of high-mobility-group box 1 (HMGB1) protein from its normal location into the circulation, are also put in context. The pathogenesis of the disease caused by falciparum malaria is then considered in the light of what has been learned about the roles of these mediators in these other diseases, as well as in malaria itself.
Figures
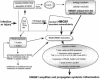
FIG. 1.
The proposed central role of HMGB1 in the amplification and perpetuation of inflammation. As well as excessive TNF and IL-1 inducing HMGB1 secretion, they can be predicted to run down cellular energy through overactivating PARP-1, thus favoring necrosis, which releases HMGB1 from the nucleus, over apoptosis, which is energy dependent and does not cause HMGB1 release. By increasing the expression of RAGE (a receptor for which HMGB1 is one of several ligands) and then activating it, HMGB1 induces a further wave of inflammatory cytokines, including the TNF and IL-1 that induced it in the first place. Thus, HMGB1 is thought to amplify and extend inflammatory reactions.
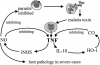
FIG. 2.
The shared ability of cytokine-induced NO and CO to inhibit TNF production and therefore provide a negative-feedback loop to inhibit the illness and pathology in cytokine-release syndromes. Reprinted from reference with permission from the publisher.
FIG. 3.
HO-1 staining of liver tissue, showing a representative field of the three cases in which death was not associated with severe systemic infection or coma (A) and and a representative field from the 37 malaria or sepsis patients who had been comatose before death. Biotin-conjugated secondary antibody and streptavidin-conjugated horseradish peroxidase from an LSAB+ kit (DAKO) were applied to sections to amplify the antigen signal for subsequent 3,3′-diaminobenzidine staining, which produces a permanent brown color. Magnification, ×400.
FIG. 4.
HO-1 staining of brain (A to C) and liver (D to F) tissue of each of the three categories used to group the 32 clinically defined cases of cerebral malaria, on the basis of their histological and immunohistochemistry findings, as CM(A), (A and D), CM(B), (B and E); and CM(C) (C and F). Panel A typifies the 11 cases with isolated or undetectable parasites or HO-1-stained monocytes in brain sections. Panels B and C are examples from the 7 and 14 cases, respectively, with increasing parasite and cellular involvement in cerebral blood vessels: Panel B shows an influx of young intraerythrocytic malaria parasites (para) but negligible monocytes, and panel C illustrates the more mature parasites, monocyte accumulations (mono), and microhemorrhages (hemorrh) typical of CM(C). All three categories showed systemic inflammation, as demonstrated by strongly staining Kupffer cells (D to F). Dilution of primary anti-HO-1 antibody (StressGen) was 1:1,000. Staining was done as described for Fig. 3. Magnification, ×200 in all cases.
Similar articles
- The pathophysiology of falciparum malaria.
Clark IA, Cowden WB. Clark IA, et al. Pharmacol Ther. 2003 Aug;99(2):221-60. doi: 10.1016/s0163-7258(03)00060-3. Pharmacol Ther. 2003. PMID: 12888113 Review. - Sickness behaviour pushed too far--the basis of the syndrome seen in severe protozoal, bacterial and viral diseases and post-trauma.
Clark IA, Budd AC, Alleva LM. Clark IA, et al. Malar J. 2008 Oct 14;7:208. doi: 10.1186/1475-2875-7-208. Malar J. 2008. PMID: 18854046 Free PMC article. Review. - Role of proinflammatory and anti-inflammatory cytokines in the immune response to Plasmodium falciparum malaria.
Torre D, Speranza F, Martegani R. Torre D, et al. Lancet Infect Dis. 2002 Dec;2(12):719-20. doi: 10.1016/s1473-3099(02)00449-8. Lancet Infect Dis. 2002. PMID: 12467687 No abstract available. - Increased levels of inflammatory mediators in children with severe Plasmodium falciparum malaria with respiratory distress.
Awandare GA, Goka B, Boeuf P, Tetteh JK, Kurtzhals JA, Behr C, Akanmori BD. Awandare GA, et al. J Infect Dis. 2006 Nov 15;194(10):1438-46. doi: 10.1086/508547. Epub 2006 Oct 10. J Infect Dis. 2006. PMID: 17054074 - Decreased circulating macrophage migration inhibitory factor (MIF) protein and blood mononuclear cell MIF transcripts in children with Plasmodium falciparum malaria.
Awandare GA, Hittner JB, Kremsner PG, Ochiel DO, Keller CC, Weinberg JB, Clark IA, Perkins DJ. Awandare GA, et al. Clin Immunol. 2006 May;119(2):219-25. doi: 10.1016/j.clim.2005.12.003. Epub 2006 Feb 7. Clin Immunol. 2006. PMID: 16461006
Cited by
- How malaria has affected the human genome and what human genetics can teach us about malaria.
Kwiatkowski DP. Kwiatkowski DP. Am J Hum Genet. 2005 Aug;77(2):171-92. doi: 10.1086/432519. Epub 2005 Jul 6. Am J Hum Genet. 2005. PMID: 16001361 Free PMC article. Review. - Outpatient upper respiratory tract viral infections in children with malaria symptoms in Western Kenya.
Waitumbi JN, Kuypers J, Anyona SB, Koros JN, Polhemus ME, Gerlach J, Steele M, Englund JA, Neuzil KM, Domingo GJ. Waitumbi JN, et al. Am J Trop Med Hyg. 2010 Nov;83(5):1010-3. doi: 10.4269/ajtmh.2010.10-0174. Am J Trop Med Hyg. 2010. PMID: 21036828 Free PMC article. - Ameliorative Effects of Dietary Ellagic Acid Against Severe Malaria Pathogenesis by Reducing Cytokine Storms and Oxidative Stress.
Mohanty S, Gupta AC, Maurya AK, Shanker K, Pal A, Bawankule DU. Mohanty S, et al. Front Pharmacol. 2021 Dec 9;12:777400. doi: 10.3389/fphar.2021.777400. eCollection 2021. Front Pharmacol. 2021. PMID: 34975479 Free PMC article. - Highly diluted medication reduces parasitemia and improves experimental infection evolution by Trypanosoma cruzi.
Aleixo DL, Ferraz FN, Ferreira EC, de Lana M, Gomes ML, de Abreu Filho BA, de Araújo SM. Aleixo DL, et al. BMC Res Notes. 2012 Jul 11;5:352. doi: 10.1186/1756-0500-5-352. BMC Res Notes. 2012. PMID: 22784664 Free PMC article. - The Nociceptive and Inflammatory Responses Induced by the Ehrlich Solid Tumor Are Changed in Mice Healed of Plasmodium berghei Strain ANKA Infection after Chloroquine Treatment.
Rodrigues Aguiar MF, Guterres MM, Benarrosh EM, Verri WA Jr, Calixto-Campos C, Dias QM. Rodrigues Aguiar MF, et al. J Parasitol Res. 2024 May 14;2024:3771926. doi: 10.1155/2024/3771926. eCollection 2024. J Parasitol Res. 2024. PMID: 38774541 Free PMC article.
References
- Abdelkarim, G. E., K. Gertz, C. Harms, J. Katchanov, U. Dirnagl, C. Szabo, and M. Endres. 2001. Protective effects of PJ34, a novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP) in in vitro and in vivo models of stroke. Int. J. Mol. Med. 7:255-260. - PubMed
- Abrahamsohn, I. A., and R. L. Coffman. 1995. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J. Immunol. 155:3955-3963. - PubMed
- Abuin, G., A. S. Couto, R. M. Delederkremer, O. L. Casal, C. Galli, W. Colli, and M. J. M. Alves. 1996. Trypanosoma cruzi: the TC-85 surface glycoprotein shed by trypomastigotes bears a modified glycosylphosphatidylinositol anchor. Exp. Parasitol. 82:290-297. - PubMed
- Adams, J. H. 1989. Cerebral infarction — its pathogenesis and interpretation. J. Pathol. 157:281-282. - PubMed
- Aderka, D., H. Holtmann, L. Toker, T. Hahn, and D. Wallach. 1986. Tumor necrosis factor induction by Sendai virus. J. Immunol. 136:2938-2942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous