NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes - PubMed (original) (raw)
NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes
Jonah R Chan et al. Neuron. 2004.
Abstract
Axons dictate whether or not they will become myelinated in both the central and peripheral nervous systems by providing signals that direct the development of myelinating glia. Here we identify the neurotrophin nerve growth factor (NGF) as a potent regulator of the axonal signals that control myelination of TrkA-expressing dorsal root ganglion neurons (DRGs). Unexpectedly, these NGF-regulated axonal signals have opposite effects on peripheral and central myelination, promoting myelination by Schwann cells but reducing myelination by oligodendrocytes. These findings indicate a novel role for growth factors in regulating the receptivity of axons to myelination and reveal that different axonal signals control central and peripheral myelination.
Figures
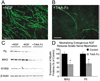
Figure 1. NGF Promotes Myelination of DRGs by Schwann Cells
(A and B) Anti-MBP immunostaining (green) of Schwann cell-DRG cocultures 6 days after addition of ascorbate in the presence of NGF (A) or the NGF scavenger TrkA-Fc (B). Scale bars equal 100 µm. (C) Western blots of cocultures probed for myelin (P0 and MAG) and Schwann cell (S100β) proteins. β-actin served as a loading control. (D) Injection of TrkA-Fc (2.5 µg) along the mouse sciatic nerve on the day of birth and 2 days later reduced the expression of the myelin proteins P0 and MAG at postnatal day 4 compared to the saline-injected contralateral nerve (protein levels assessed by Western blot, n = 16 per condition, % saline-injected control ± SEM, *p < 0.003, unpaired t test).
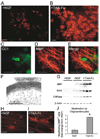
Figure 2. NGF Inhibits Myelination of DRGs by Oligodendrocytes
(A and B) Anti-MBP immunostaining (red) of OPC-DRG cocultures 14 days after seeding OPCs in the presence of NGF (A) or the NGF scavenger TrkA-Fc (B). Scale bars equal 200 µm. (C–E) A higher-magnification view of a myelinating oligodendrocyte in an OPC-DRG co-culture double-labeled with CC1 (C) and anti-MBP (D). Scale bars equal 100 µm. (F) The presence of compact myelin in these cocultures was confirmed by electron microscopy. (G) Western blots of OPC-DRG cocultures probed for myelin (MBP, MAG) and oligodendrocyte (CNPase) proteins indicate that NGF reduces oligodendrocyte maturation. β-actin served as a loading control. (H–J) NGF also limits myelination by differentiated oligodendrocytes. Cocultures of DRGs seeded with acutely purified oligodendrocytes examined byanti-MBP immunostaining (red) after 3 days. Scale bars equal 200 µm. (H) In the presence of NGF, many MBP-positive cells failed to extend processes, while others extended processes that failed to myelinate. (I) In the presence of TrkA-Fc, many oligodendrocytes myelinated DRG axons only 3 days after seeding. (J) Myelinating GalC+ A2B5− oligodendrocytes were quantified by counting the proportion of MBP+ cells with at least two myelinating processes (16 fields/coverslip, 6 coverslips/condition, fold-change from NGF condition ± SD, *p < 0.001, unpaired t test).
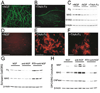
Figure 3. TrkA, not p75NTR, Mediates the Effects of NGF on Myelination
(A–C) p75NTR is not required for the NGF-mediated enhancement of Schwann cell myelination. Dissociated DRG explants from p75−/− mice fail to myelinate robustly without NGF (B), as determined by immunostaining for MBP (green) and Western blots (C) for myelin and Schwann cell proteins (P0, MAG, and S100β). Scale bars equal 100 µm. (D–F) Glial p75NTR is not required for the NGF-mediated inhibition of oligodendrocyte myelination. (D) No MBP-positive myelin (red) was observed in the presence of NGF in these xeno-cultures of rat DRGs with p75NTR-null mouse OPCs (n = 6). (E) Significant myelination was always seen in the presence of TrkA-Fc (n = 6). (F) Higher-magnification view of p75NTR-null myelinating oligodendrocytes. (G and H) TrkA-activating antibodies (RTA, 50 µg/ml) are sufficient to mimic NGF in both types of myelinating cocultures. Anti-NGF neutralizing antibodies were added to block endogenous NGF, and mouse and rabbit IgG were used as controls for anti-NGF and RTA, respectively. (G) Western blots of Schwann cell-DRG cocultures for Schwann cell and myelin proteins. (H) Western blots of OPC-DRG cocultures for oligodendrocyte and myelin proteins.
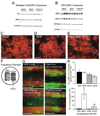
Figure 4. The Effects of NGF Are Mediated by Changes to the Axonal Signals that Control Myelination of TrkA-Positive DRGs (A–E) The effects of NGF on myelination require TrkA-positive DRGs.
(A–E) The effects of NGF on myelination require TrkA-positive DRGs. (A and B) No effects of NGF or TrkA-Fc on the expression of myelin proteins in cocultures of Schwann cells (A) or cocultures of OPCs (B) with BDNF-dependent DRGs, as assessed by Western blot. (C–E) No effects of NGF or TrkA-Fc on the numbers of oligodendrocytes or myelin internodes in cocultures of OPCs with BDNF-dependent DRGs, as assessed by immunostaining for MBP (red). (F–H) Exposure of NGF to TrkA-expressing DRG soma or axons results in inhibition of oligodendrocyte myelination. (F) TrkA-expressing DRGs in the somal compartment of Campenot chambers extend axons through a vacuum grease seal to the neighboring chamber. OPCs were seeded in the axonal chamber only. (G) Extensive myelination (MBP; green) of TrkA (red)-expressing axons is only seen when TrkA-Fc is present in both the axonal and somal compartments. (H) The number of nonmyelinating MBP-positive oligodendrocytes and myelin segments were quantified by counting 6 fields/chamber, 4 chambers/condition (cells or myelin segments/field ± SEM). *p < 0.01 versus NGF in both compartments and † p < 0.05 versus TrkA in both compartments (Student-Newman-Keuls post hoc comparison after one-way ANOVA).
Comment in
- New roles for an old molecule in axon-glial interaction.
Brophy PJ. Brophy PJ. Neuron. 2004 Jul 22;43(2):154-5. doi: 10.1016/j.neuron.2004.07.005. Neuron. 2004. PMID: 15260949
Similar articles
- Effects of delayed myelination by oligodendrocytes and Schwann cells on the macromolecular structure of axonal membrane in rat spinal cord.
Black JA, Waxman SG, Sims TJ, Gilmore SA. Black JA, et al. J Neurocytol. 1986 Dec;15(6):745-61. doi: 10.1007/BF01625192. J Neurocytol. 1986. PMID: 3819778 - The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination.
Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. Einheber S, et al. J Cell Biol. 1997 Dec 15;139(6):1495-506. doi: 10.1083/jcb.139.6.1495. J Cell Biol. 1997. PMID: 9396755 Free PMC article. - In Vitro Myelination of Peripheral Axons in a Coculture of Rat Dorsal Root Ganglion Explants and Schwann Cells.
Blusch A, Sgodzai M, Rilke N, Motte J, König J, Pitarokoili K, Grüter T. Blusch A, et al. J Vis Exp. 2023 Feb 10;(192). doi: 10.3791/64768. J Vis Exp. 2023. PMID: 36847366 - How histone deacetylases control myelination.
Jacob C, Lebrun-Julien F, Suter U. Jacob C, et al. Mol Neurobiol. 2011 Dec;44(3):303-12. doi: 10.1007/s12035-011-8198-9. Epub 2011 Aug 23. Mol Neurobiol. 2011. PMID: 21861092 Review. - Architecting the myelin landscape.
Osso LA, Chan JR. Osso LA, et al. Curr Opin Neurobiol. 2017 Dec;47:1-7. doi: 10.1016/j.conb.2017.06.005. Epub 2017 Jul 11. Curr Opin Neurobiol. 2017. PMID: 28709021 Free PMC article. Review.
Cited by
- Role of glial cells in axonal regeneration.
Toy D, Namgung U. Toy D, et al. Exp Neurobiol. 2013 Jun;22(2):68-76. doi: 10.5607/en.2013.22.2.68. Epub 2013 Jun 27. Exp Neurobiol. 2013. PMID: 23833555 Free PMC article. - Stage-specific deletion of Olig2 conveys opposing functions on differentiation and maturation of oligodendrocytes.
Mei F, Wang H, Liu S, Niu J, Wang L, He Y, Etxeberria A, Chan JR, Xiao L. Mei F, et al. J Neurosci. 2013 May 8;33(19):8454-62. doi: 10.1523/JNEUROSCI.2453-12.2013. J Neurosci. 2013. PMID: 23658182 Free PMC article. - Fabrication, characterization and cellular compatibility of poly(hydroxy alkanoate) composite nanofibrous scaffolds for nerve tissue engineering.
Masaeli E, Morshed M, Nasr-Esfahani MH, Sadri S, Hilderink J, van Apeldoorn A, van Blitterswijk CA, Moroni L. Masaeli E, et al. PLoS One. 2013;8(2):e57157. doi: 10.1371/journal.pone.0057157. Epub 2013 Feb 27. PLoS One. 2013. PMID: 23468923 Free PMC article. - NGF regulates the expression of axonal LINGO-1 to inhibit oligodendrocyte differentiation and myelination.
Lee X, Yang Z, Shao Z, Rosenberg SS, Levesque M, Pepinsky RB, Qiu M, Miller RH, Chan JR, Mi S. Lee X, et al. J Neurosci. 2007 Jan 3;27(1):220-5. doi: 10.1523/JNEUROSCI.4175-06.2007. J Neurosci. 2007. PMID: 17202489 Free PMC article. - Expression of NGF and GDNF family members and their receptors during peripheral nerve development and differentiation of Schwann cells in vitro.
Piirsoo M, Kaljas A, Tamm K, Timmusk T. Piirsoo M, et al. Neurosci Lett. 2010 Jan 18;469(1):135-40. doi: 10.1016/j.neulet.2009.11.060. Epub 2009 Nov 26. Neurosci Lett. 2010. PMID: 19944743 Free PMC article.
References
- Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. - PubMed
- Bahr M, Hopkins JM, Bunge RP. In vitro myelination of regenerating adult rat retinal ganglion cell axons by Schwann cells. Glia. 1991;4:529–533. - PubMed
- Bansal R, Pfeiffer SE. Developmental expression of 2′,3′-cyclic nucleotide 3′-phosphohydrolase in dissociated fetal rat brain cultures and rat brain. J. Neurosci. Res. 1985;14:21–34. - PubMed
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. - PubMed
- Barres BA, Raff MC, Gaese F, Bartke I, Dechant G, Barde YA. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature. 1994;367:371–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS004270/NS/NINDS NIH HHS/United States
- NS04270/NS/NINDS NIH HHS/United States
- R01 EY010257/EY/NEI NIH HHS/United States
- R01 EY10257/EY/NEI NIH HHS/United States
- P01 NS016033/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources