Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system - PubMed (original) (raw)
Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system
Biljana Georgievska et al. J Neurosci. 2004.
Abstract
The effects of continuous glial cell line-derived neurotrophic factor (GDNF) overexpression in the intact nigrostriatal dopamine (DA) system was studied using recombinant lentiviral (rLV) vector delivery of GDNF to the striatum or substantia nigra (SN) in the rat. Intrastriatal delivery of rLV-GDNF resulted in significant overexpression of GDNF in the striatum (2-4 ng/mg tissue) and anterograde transport of GDNF protein to the SN. Striatal rLV-GDNF delivery initially induced an increase in DA turnover (1-6 weeks), accompanied by significant contralateral turning in response to amphetamine, suggesting an enhancement of the DA system on the injected side. Starting 6 weeks after continuous GDNF delivery, we observed a selective downregulation of tyrosine hydroxylase (TH) protein (approximately 70%) that was maintained until the end of the experiment (24 weeks). A similar effect was observed when rLV-GDNF was injected into the SN. The magnitude of TH downregulation was related to the level of GDNF expression and was most pronounced in animals in which the striatal GDNF level exceeded 0.7 ng/mg tissue. The decreased TH protein levels were associated with similar reductions in the in vitro TH enzyme activity (approximately 70%); however, in vivo L-3,4-dihydroxyphenylalanine production rate and DA tissue levels were maintained at normal levels. The results indicate that downregulation of TH protein reflects a compensatory effect in response to continuous GDNF stimulation of the DA neurons mediated by a combination of overactivity at the DA synapse and a direct GDNF-induced action on TH gene expression. This compensatory mechanism is proposed to maintain long-term DA neuron function within the normal range.
Figures
Figure 1.
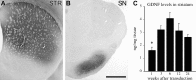
Time course of GDNF expression after intrastriatal injections of rLV-GDNF (1 × 105 TU). A, B, Photomicrographs of sections through striatum (A) and midbrain (B) immunostained for GDNF at 6 weeks after rLV-GDNF injection, illustrating the widespread distribution of GDNF in the striatum and anterograde transport of GDNF protein to the SN pars reticulata region. Scale bar: (in B) A, B, 1 mm. C, Determination of GDNF tissue levels (nanograms per milligram of tissue) as measured by ELISA in the striatum at different time points after rLV-GDNF injection. GDNF was detected at all time points (1-24 weeks), and the levels were between 1.6 and 4.2 ng/mg tissue. The level at 1 week after surgery was significantly lower than the level measured at 6 weeks (*p < 0.01). Values give means ± SEM.
Figure 2.
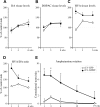
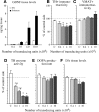
Changes in striatal DA turnover and amphetamine-induced rotation over time. _A_-C, Striatal tissue levels of DA (A) and DOPAC (B) were unchanged in the rLV-GDNF-treated animals, whereas the HVA tissue levels (C) were significantly increased at 1 week after rLV injection. D, The HVA/DA ratio (reflecting the DA turnover and release) was significantly (p < 0.0001) increased in the rLV-GDNF group at all time points; however, the DA turn over decreased over time. E, Injection of amphetamine induced a significant (p < 0.01) contralateral turning in the GDNF-treated animals at 1, 3, and 6 weeks (≈5-7 turns/min). At the later time points, the turning rate declined in the rLV-GDNF group and was no longer different from the rLV-GFP-treated control animals at 12 and 19 weeks (≈1-3 turns/min). Values give means ± SEM. An asterisk indicates a significant difference from the rLV-GFP group.
Figure 3.
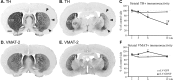
Effects of striatal rLV-GDNF delivery on TH and VMAT-2 immunoreactivity in the striatum. A, B, Photomicrographs of coronal sections through the rostral (A) and caudal (B) striatum, immunostained for TH at 6 weeks after rLV injection. Note the reduced TH immunoreactivity on the injected (right) side (arrowheads). C, Densitometry measurements of the striatal fiber staining at 1, 3, 6, and 12 weeks showed a significant decrease (p < 0.001) in TH-positive immunoreactivity on the injected side in the rLV-GDNF group at 6 and 12 weeks compared with the rLV-GFP-treated group. The reduction in TH-positive immunoreactivity was most pronounced in the caudal striatum (_B_) and to a lesser extent in the rostral striatum (_A_). _D, E_, Photomicrographs of adjacent coronal sections immunostained for VMAT-2 at 6 weeks after rLV injection. _F_, The VMAT-2-positive immunoreactivity was not significantly affected at any time point (_p_ > 0.05). Values give means ± SEM. An asterisk indicates a significant difference from the rLV-GFP-treated group; a number sign indicates a significant difference between 3 and 6 weeks in the rLV-GDNF group (p < 0.0001). Scale bar: (in A) A, B, D, E, 1 mm. S, Septum; GP, globus pallidus; STR, striatum.
Figure 4.
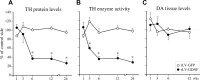
Biochemical analyses in striatal tissue samples at different time points after rLV-GDNF vector injection in the striatum (1 × 105 TU). A, TH protein levels were determined by Western blot analysis and showed significant reductions (p < 0.0001) at 6 weeks (36.1 ± 3.5%) in the rLV-GDNF group; these reductions were maintained at the later time points (12 and 24 weeks). _B_, Measurement of TH enzyme activity showed a significant decrease (_p_ < 0.0001) at 3 weeks (58.6 ± 2.1%) in the rLV-GDNF group. The TH enzyme activity was further reduced at 6 weeks (34.4 ± 6.4%) and maintained at this low level at 12 and 24 weeks. _C_, Determination of DA levels did not reveal any significant changes between the groups at any time point (_p_ > 0.05). Values give means ± SEM. An asterisk indicates a significant difference from the rLV-GFP group.
Figure 5.
Histological and biochemical analyses at 6 weeks after injection of different doses of rLV-GDNF in the striatum (0.4-10 × 104 TU). A, GDNF tissue levels (nanograms per milligram of tissue) were determined by ELISA in striatum and SN. The GDNF levels in the striatum ranged from 0.10 ± 0.03 ng/mg tissue in the 0.4 × 104 TU group to 7.4 ± 1.1 ng/mg tissue in the 10 × 104 TU group. The GDNF levels detected in the SN were 0.06 ± 0.01 ng/mg tissue in the lowest-dose group and increased to 1.01 ± 0.14 ng/mg tissue in the highest-dose group. B, Densitometry measurements of striatal TH-positive immunoreactivity showed significant reductions (p < 0.01) in the rLV-GDNF groups receiving the higher doses (1-10 × 104 TU), whereas it was not changed in the group receiving the lowest dose (0.4 × 104 TU). _C_, Densitometry measurements of VMAT-2 immunoreactivity in the striatum did not show any significant differences between groups (_p_ > 0.05). D, TH enzyme activity measured in striatal tissue samples showed significant reductions (p < 0.0001) in all GDNF-treated groups compared with the rLV-GFP-treated control group. The magnitude of reduction was significantly higher in the rLV-GDNF groups receiving 4-10 × 104 TU (-72 ± 3%) compared with the lower-dose groups (-43 ± 4%). _E_, Determination of the _in vivo_ DOPA production rate did not show any significant changes (_p_ > 0.05) in the rLV-GDNF groups. F, Determination of DA levels in the striatal tissue samples showed a significant reduction (p < 0.05) in the rLV-GDNF group receiving 4 × 104 TU, whereas the other groups maintained their DA levels at a close to normal level. Values give means ± SEM. An asterisk indicates a significant difference from the rLV-GFP control group; a number sign indicates a significant difference from the other rLV-GDNF-treated groups. C, Control.
Figure 6.
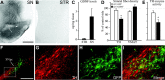
Histological and biochemical analyses at 6 weeks after injection of rLV-GDNF in the SN (1 × 105 TU). A, B, Photomicrographs of sections through the midbrain and striatum immunostained for GDNF showed that GDNF protein was widely spread in the midbrain on the injected side (A), whereas no GDNF could be visualized in the ipsilateral striatum (B). C, Determination of GDNF levels by ELISA in tissue samples from the striatum (0.09 ± 0.04 ng/mg tissue) and SN (2.4 ± 0.8 ng/mg tissue) at 6 weeks after rLV injection. D, Densitometry measurements of the striatal fiber staining showed a significant decrease (p < 0.01) in TH-positive immunoreactivity on the injected side in the rLV-GDNF group (66.4 ± 3.8%), whereas VMAT-2 immunoreactivity was unchanged (_p_ > 0.05). E, TH enzyme activity in the striatal tissue samples was significantly reduced (p < 0.05) in the rLV-GDNF group._F_-I, Double labeling of TH (red) and GFP (green) in a section through the midbrain of an animal injected with rLV-GFP in the SN. TH-positive cells in the SN (G) were rarely colabeled with GFP (H) in the SN (I). The box in F depicts the approximate area from which the high-power pictures are taken. Scale bars: (in A) A, B, 1 mm; F, 500 μm; (in F) _G_-I, 250 μm. Values give means ± SEM. An asterisk indicates a significant difference from the rLV-GFP group. SNpc, Substantia nigra pars compacta.
Similar articles
- Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system.
Rosenblad C, Georgievska B, Kirik D. Rosenblad C, et al. Eur J Neurosci. 2003 Jan;17(2):260-70. doi: 10.1046/j.1460-9568.2003.02456.x. Eur J Neurosci. 2003. PMID: 12542662 - Adenoviral vector-mediated delivery of glial cell line-derived neurotrophic factor provides neuroprotection in the aged parkinsonian rat.
Connor B. Connor B. Clin Exp Pharmacol Physiol. 2001 Nov;28(11):896-900. doi: 10.1046/j.1440-1681.2001.03544.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11703392 Review. - Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model.
Björklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ. Björklund A, et al. Brain Res. 2000 Dec 15;886(1-2):82-98. doi: 10.1016/s0006-8993(00)02915-2. Brain Res. 2000. PMID: 11119690 Review.
Cited by
- Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson's disease.
Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Annett L, Kirik D. Eslamboli A, et al. J Neurosci. 2005 Jan 26;25(4):769-77. doi: 10.1523/JNEUROSCI.4421-04.2005. J Neurosci. 2005. PMID: 15673656 Free PMC article. - Efficient Expression of Igf-1 from Lentiviral Vectors Protects In Vitro but Does Not Mediate Behavioral Recovery of a Parkinsonian Lesion in Rats.
Lu-Nguyen NB, Broadstock M, Yáñez-Muñoz RJ. Lu-Nguyen NB, et al. Hum Gene Ther. 2015 Nov;26(11):719-33. doi: 10.1089/hum.2015.016. Epub 2015 Oct 1. Hum Gene Ther. 2015. PMID: 26222254 Free PMC article. - Transgenic expression of human glial cell line-derived neurotrophic factor from integration-deficient lentiviral vectors is neuroprotective in a rodent model of Parkinson's disease.
Lu-Nguyen NB, Broadstock M, Schliesser MG, Bartholomae CC, von Kalle C, Schmidt M, Yáñez-Muñoz RJ. Lu-Nguyen NB, et al. Hum Gene Ther. 2014 Jul;25(7):631-41. doi: 10.1089/hum.2014.003. Epub 2014 Apr 15. Hum Gene Ther. 2014. PMID: 24635742 Free PMC article. - Two-fold elevation of endogenous GDNF levels in mice improves motor coordination without causing side-effects.
Mätlik K, Võikar V, Vilenius C, Kulesskaya N, Andressoo JO. Mätlik K, et al. Sci Rep. 2018 Aug 8;8(1):11861. doi: 10.1038/s41598-018-29988-1. Sci Rep. 2018. PMID: 30089897 Free PMC article. - Comparison of the capability of GDNF, BDNF, or both, to protect nigrostriatal neurons in a rat model of Parkinson's disease.
Sun M, Kong L, Wang X, Lu XG, Gao Q, Geller AI. Sun M, et al. Brain Res. 2005 Aug 9;1052(2):119-29. doi: 10.1016/j.brainres.2005.05.072. Brain Res. 2005. PMID: 16018990 Free PMC article.
References
- Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, Rosenthal A, Hefti F (1995) Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature 373: 339-341. - PubMed
- Bilang-Bleuel A, Revah F, Colin P, Locquet I, Robert JJ, Mallet J, Horellou P (1997) Intrastriatal injection of an adenoviral vector expressing glial-cell-line-derived neurotrophic factor prevents dopaminergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease. Proc Natl Acad Sci USA 94: 8818-8823. - PMC - PubMed
- Björklund A, Rosenblad C, Winkler C, Kirik D (1997) Studies on neuroprotective and regenerative effects of GDNF in a partial lesion model of Parkinson's disease. Neurobiol Dis 4: 186-200. - PubMed
- Björklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ (2000) Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res 886: 82-98. - PubMed
- Bowenkamp KE, Ujhelyi L, Cline EJ, Bickford PC (2000) Effects of intrastriatal GDNF on motor coordination and striatal electrophysiology in aged F344 rats. Neurobiol Aging 21: 117-124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources