Motor skill learning depends on protein synthesis in motor cortex after training - PubMed (original) (raw)
Motor skill learning depends on protein synthesis in motor cortex after training
Andreas R Luft et al. J Neurosci. 2004.
Abstract
The role of protein synthesis in memory consolidation is well established for hippocampus-dependent learning and synaptic plasticity. Whether protein synthesis is required for motor skill learning is unknown. We hypothesized that skill learning is interrupted by protein synthesis inhibition (PSI). We intended to test whether local protein synthesis in motor cortex or cerebellum is required during skill acquisition and consolidation. Anisomycin (ANI; 100 microg/microl in 1 microl of PBS) injected into motor cortex, posterior parietal cortex, or cerebellum produced 84.0 +/- 1.44% (mean +/- SEM), 85.9 +/- 2.31%, and 87.3 +/- 0.17% of PSI 60 min after administration, respectively. In motor cortex, protein synthesis was still reduced at 24 hr (72.0 +/- 4.68% PSI) but normalized at 48 hr after a second injection given 24 hr after the first. To test for the effects of PSI on learning of a skilled reaching task, ANI was injected into motor cortex contralateral to the trained limb or into ipsilateral cerebellum immediately after daily training sessions 1 and 2. Two control groups received motor cortex injections of vehicle or ANI injections into contralateral parietal cortex. Control and cerebellar animals showed a sigmoid learning curve, which plateaued after day 4. PSI in motor cortex significantly reduced learning during days 1-4. Thereafter, when protein synthesis normalized, learning was reinitiated. ANI injections into motor cortex did not induce a motor deficit, because animals injected during the performance plateau did not deteriorate. This demonstrates that motor skill learning depends on de novo synthesis of proteins in motor cortex after training.
Figures
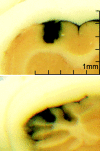
Figure 1.
Spread of ink injected into motor cortex and cerebellum (experiment 2). India ink was used as a visual stimulant to estimate the initial spread of ANI in cortex (top) and in cerebellum (bottom). Sections from one exemplary animal are shown.
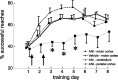
Figure 2.
Effects of protein synthesis inhibition on motor skill learning (experiment 3). Inhibition of protein synthesis in motor cortex by local injection of ANI at the time points indicated by arrows impairs motor skill acquisition for 4 d. In contrast, ANI injections into cerebellum or parietal cortex do not affect motor skill learning (*p < 0.05). After protein synthesis is restored (day 4), learning resumes but at a lower rate (see Discussion).
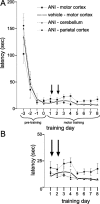
Figure 3.
Effects of PSI on latencies between pellet removal and door opening (experiment 3). A, During pretraining (sessions 3-0, pellet accessible by tongue) and subsequent forelimb reach training (sessions 1-8), latencies decrease exponentially. PSI in motor cortex (ANI-motor cortex group) does slightly but nonsignificantly increase latencies. B shows this increase on a stretched y-scale. Time points of ANI injections are indicated by arrows.
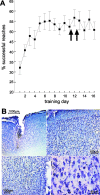
Figure 4.
Effects of PSI on motor performance (experiment 4). A, To test whether PSI in motor cortex induces motor deficits, animals were injected with anisomycin during the plateau phase of performance (immediately after sessions 11 and 12). No performance decline is observed. B, Motor cortical histology (cresyl violet) obtained from one exemplary animal implanted with the cannula system and injected twice with ANI provides no evidence for cortical injury apart from the needle tract and a small area of hemorrhagic transformation just below the tip of the cannula.
Similar articles
- Motor skill learning depends on protein synthesis in the dorsal striatum after training.
Wächter T, Röhrich S, Frank A, Molina-Luna K, Pekanovic A, Hertler B, Schubring-Giese M, Luft AR. Wächter T, et al. Exp Brain Res. 2010 Jan;200(3-4):319-23. doi: 10.1007/s00221-009-2027-7. Epub 2009 Oct 13. Exp Brain Res. 2010. PMID: 19823812 - Protein Synthesis Inhibition in the Peri-Infarct Cortex Slows Motor Recovery in Rats.
Schubring-Giese M, Leemburg S, Luft AR, Hosp JA. Schubring-Giese M, et al. PLoS One. 2016 Jun 17;11(6):e0157859. doi: 10.1371/journal.pone.0157859. eCollection 2016. PLoS One. 2016. PMID: 27314672 Free PMC article. - Region and task-specific activation of Arc in primary motor cortex of rats following motor skill learning.
Hosp JA, Mann S, Wegenast-Braun BM, Calhoun ME, Luft AR. Hosp JA, et al. Neuroscience. 2013 Oct 10;250:557-64. doi: 10.1016/j.neuroscience.2013.06.060. Epub 2013 Jul 19. Neuroscience. 2013. PMID: 23876329 - Stages of motor skill learning.
Luft AR, Buitrago MM. Luft AR, et al. Mol Neurobiol. 2005 Dec;32(3):205-16. doi: 10.1385/MN:32:3:205. Mol Neurobiol. 2005. PMID: 16385137 Review. - Learning strategies: a synthesis and conceptual model.
Hattie JAC, Donoghue GM. Hattie JAC, et al. NPJ Sci Learn. 2016 Aug 10;1:16013. doi: 10.1038/npjscilearn.2016.13. eCollection 2016. NPJ Sci Learn. 2016. PMID: 30792898 Free PMC article. Review.
Cited by
- The effect of reward on motor learning: different stage, different effect.
Zhao J, Zhang G, Xu D. Zhao J, et al. Front Hum Neurosci. 2024 Mar 12;18:1381935. doi: 10.3389/fnhum.2024.1381935. eCollection 2024. Front Hum Neurosci. 2024. PMID: 38532789 Free PMC article. Review. - Exploration biases forelimb reaching strategies.
Mosberger AC, Sibener LJ, Chen TX, Rodrigues HFM, Hormigo R, Ingram JN, Athalye VR, Tabachnik T, Wolpert DM, Murray JM, Costa RM. Mosberger AC, et al. Cell Rep. 2024 Apr 23;43(4):113958. doi: 10.1016/j.celrep.2024.113958. Epub 2024 Mar 22. Cell Rep. 2024. PMID: 38520691 Free PMC article. - A step toward restoring hand functions in patients with multiple sclerosis-a study protocol.
Zoghi M, Jaberzadeh S. Zoghi M, et al. Front Rehabil Sci. 2023 Jun 14;4:1053577. doi: 10.3389/fresc.2023.1053577. eCollection 2023. Front Rehabil Sci. 2023. PMID: 37387732 Free PMC article. - Endogenous dopamine transmission is crucial for motor skill recovery after stroke.
Vitrac C, Nallet-Khosrofian L, Iijima M, Rioult-Pedotti MS, Luft A. Vitrac C, et al. IBRO Neurosci Rep. 2022 Jun 2;13:15-21. doi: 10.1016/j.ibneur.2022.05.008. eCollection 2022 Dec. IBRO Neurosci Rep. 2022. PMID: 35707766 Free PMC article. - Physical exercise rescues cocaine-evoked synaptic deficits in motor cortex.
Cheng T, Huang XD, Hu XF, Wang SQ, Chen K, Wei JA, Yan L, So KF, Yuan TF, Zhang L. Cheng T, et al. Mol Psychiatry. 2021 Nov;26(11):6187-6197. doi: 10.1038/s41380-021-01336-2. Epub 2021 Oct 22. Mol Psychiatry. 2021. PMID: 34686765
References
- Arshavsky YI (2003) Long-term memory: does it have a structural or chemical basis? Trends Neurosci 26: 465-466. - PubMed
- Atkins MJ, Apps R (1997) Somatotopical organisation within the climbing fibre projection to the paramedian lobule and copula pyramidis of the rat cerebellum. J Comp Neurol 389: 249-263. - PubMed
- Berman DE, Dudai Y (2001) Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science 291: 2417-2419. - PubMed
- Bickford P (1995) Aging and motor learning: a possible role for norepinephrine in cerebellar plasticity. Rev Neurosci 6: 35-46. - PubMed
- Brown IE, Bower JM (2001) Congruence of mossy fiber and climbing fiber tactile projections in the lateral hemispheres of the rat cerebellum. J Comp Neurol 429: 59-70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources