Shaggy, the homolog of glycogen synthase kinase 3, controls neuromuscular junction growth in Drosophila - PubMed (original) (raw)
Shaggy, the homolog of glycogen synthase kinase 3, controls neuromuscular junction growth in Drosophila
Bénédicte Franco et al. J Neurosci. 2004.
Abstract
A protein-trap screen using the Drosophila neuromuscular junction (NMJ) as a model synapse was performed to identify genes that control synaptic structure or plasticity. We found that Shaggy (Sgg), the Drosophila homolog of the mammalian glycogen synthase kinases 3 alpha and beta, two serine-threonine kinases, was concentrated at this synapse. Using various combinations of mutant alleles of shaggy, we found that Shaggy negatively controlled the NMJ growth. Moreover, tissue-specific expression of a dominant-negative Sgg indicated that this kinase is required in the motoneuron, but not in the muscle, to control NMJ growth. Finally, we show that Sgg controlled the microtubule cytoskeleton dynamics in the motoneuron and that Futsch, a microtubule-associated protein, was required for Shaggy function on synaptic growth.
Figures
Figure 3.
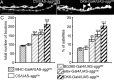
Overexpression of a dominant-negative Sgg with different Gal4 drivers. A, B, Illustration of the overgrowth phenotype when SggDN is expressed presynaptically: anti-HRP staining of a muscle 6/7 NMJ of a control female larva (CS/UAS-sggDN) (A) or a female overexpressing SggDN presynaptically (OK6-Gal4/UAS-sggDN) (B). Arrowheads indicate satellite boutons. Scale bar, 11 μm. C, Quantification of the total bouton number and the percentage of satellite boutons of control and SggDN overexpressing flies. n = 25, 26, 18, 31, and 14 for crosses with CS, MHC-Gal4, BG380-Gal4, elav-Gal4, and OK6-Gal4, respectively. **p < 0.01; ***p < 0.001.
Figure 4.
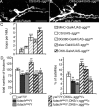
Effect of SggDN overexpression on microtubule dynamics and genetic interaction with futsch. A, B, Illustration of the increase in the number of microtubule loops when SggDN is expressed presynaptically: anti-Futsch staining of a muscle 13 NMJ on a control female larva (CS/UAS-sggDN) (A) or a female overexpressing SggDN presynaptically (OK6-Gal4/UAS-sggDN) (B). Arrows indicate loops within satellite boutons. Scale bar, 10 μm. C, Quantification of the number of microtubule loops in control larvae versus larvae expressing the SggDN protein. n = 15, 9, 9, 15, and 6 for UAS-sggDN crosses with CS, MHC-Gal4, BG380-Gal4, elav-Gal4, and OK6-Gal4. **p < 0.01; ***p < 0.001. D, Genetic interaction between SggDN expressed presynaptically and futschN94 or futschK68. Quantification of boutons number and percentage of satellite boutons of larvae expressing SggDN in the presynapse in a wild-type, futschN94, and futschK68 genetic background. n = 29 for ywCS/Y, 22 for futschN94/Y and futschK68/Y, 28 for ywCS/Y;OK6/+;sggDN/+, 30 for futschN94/Y;OK6/+;sggDN/+, and 22 for futschK68/Y;OK6/+;sggDN/+. ***p < 0.001 for statistical significance versus ywCS/Y; OK6/+; sggDN/+; #p < 0.05; ##p < 0.01; ###p < 0.001 for statistical significance versus ywCS/Y.
Figure 1.
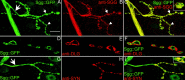
Synaptic localization of Sgg::GFP and Shaggy. GFP fluorescence (A, D, G) and anti-SGG (B), anti-DLG (E), and anti-Synapsin (SYN) (H) immunostainings on 105-1 protein trap line. All NMJs shown are located on muscle 4, but the same results could be observed on any other body-wall muscle. Color merges are shown (C, F, I), with higher magnification of one varicosity in C and I. Small arrows indicate type Ib boutons, and arrowheads indicate type Is boutons. Large arrows indicate the nerve branch. Scale bars: _A_-_C, G_-I, 20 μm; _D_-F, 10 μm.
Figure 2.
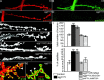
Morphological phenotype of sgg mutants. A-D, Anti-HRP (A, C) and anti-SGG (B, D) immunostainings on a CS male larva (A, B) or sggK22/Y larva (C, D). E-H, Illustration of the overgrowth phenotype in sggK22/Y (F) or sggK22/Df(1)64j4 (G) mutant larvae. This phenotype is rescued to the wild-type range (E) by the addition of an sgg minigene (mini10) in the genome of sggK22 mutants (H). The NMJs of muscle 6/7 are visualized with an anti-HRP staining. I, Quantification of the total number of Ib boutons and the percentage of satellite boutons at muscles 6/7 NMJs of CS male (n = 12), sggK22/Y (n = 25), sggK22/Df(1)64j4 (n = 29), sggK22/Y;mini10/+ (n = 14), and ywCS/+;mini10/+ (n = 13) larvae. Statistical comparisons to the CS control: *p < 0.05; **p < 0.01; ***p < 0.001. J, Anti-SYN (green) and anti-HRP (red) double staining on an sggK22/Y muscle 13 NMJ. K, Anti-DLG (green) and anti-HRP (red) double staining on an sggK22/Y muscle 13 NMJ. In all images, arrowheads indicate satellite boutons. Scale bars: _A_-D, 20 μm; _F_-H, 11 μm; J, K, 14 μm.
Similar articles
- The Drosophila microtubule associated protein Futsch is phosphorylated by Shaggy/Zeste-white 3 at an homologous GSK3beta phosphorylation site in MAP1B.
Gögel S, Wakefield S, Tear G, Klämbt C, Gordon-Weeks PR. Gögel S, et al. Mol Cell Neurosci. 2006 Oct;33(2):188-99. doi: 10.1016/j.mcn.2006.07.004. Epub 2006 Sep 1. Mol Cell Neurosci. 2006. PMID: 16949836 - The hangover gene negatively regulates bouton addition at the Drosophila neuromuscular junction.
Schwenkert I, Eltrop R, Funk N, Steinert JR, Schuster CM, Scholz H. Schwenkert I, et al. Mech Dev. 2008 Aug;125(8):700-11. doi: 10.1016/j.mod.2008.04.004. Epub 2008 Apr 27. Mech Dev. 2008. PMID: 18524547 - Functional studies of shaggy/glycogen synthase kinase 3 phosphorylation sites in Drosophila melanogaster.
Papadopoulou D, Bianchi MW, Bourouis M. Papadopoulou D, et al. Mol Cell Biol. 2004 Jun;24(11):4909-19. doi: 10.1128/MCB.24.11.4909-4919.2004. Mol Cell Biol. 2004. PMID: 15143183 Free PMC article. - Development and plasticity of the Drosophila larval neuromuscular junction.
Menon KP, Carrillo RA, Zinn K. Menon KP, et al. Wiley Interdiscip Rev Dev Biol. 2013 Sep-Oct;2(5):647-70. doi: 10.1002/wdev.108. Epub 2013 Feb 5. Wiley Interdiscip Rev Dev Biol. 2013. PMID: 24014452 Free PMC article. Review. - Neuromuscular development in Drosophila: insights from embryos and pupae.
Fernandes J, Keshishian H. Fernandes J, et al. Curr Opin Neurobiol. 1995 Feb;5(1):10-8. doi: 10.1016/0959-4388(95)80081-6. Curr Opin Neurobiol. 1995. PMID: 7772998 Review.
Cited by
- Hierarchical microtubule organization controls axon caliber and transport and determines synaptic structure and stability.
Stephan R, Goellner B, Moreno E, Frank CA, Hugenschmidt T, Genoud C, Aberle H, Pielage J. Stephan R, et al. Dev Cell. 2015 Apr 6;33(1):5-21. doi: 10.1016/j.devcel.2015.02.003. Epub 2015 Mar 19. Dev Cell. 2015. PMID: 25800091 Free PMC article. - Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function.
Sherwood NT, Sun Q, Xue M, Zhang B, Zinn K. Sherwood NT, et al. PLoS Biol. 2004 Dec;2(12):e429. doi: 10.1371/journal.pbio.0020429. Epub 2004 Nov 30. PLoS Biol. 2004. PMID: 15562320 Free PMC article. - Erect wing regulates synaptic growth in Drosophila by integration of multiple signaling pathways.
Haussmann IU, White K, Soller M. Haussmann IU, et al. Genome Biol. 2008 Apr 17;9(4):R73. doi: 10.1186/gb-2008-9-4-r73. Genome Biol. 2008. PMID: 18419806 Free PMC article. - GSK3 as a Sensor Determining Cell Fate in the Brain.
Cole AR. Cole AR. Front Mol Neurosci. 2012 Feb 9;5:4. doi: 10.3389/fnmol.2012.00004. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22363258 Free PMC article. - Neuronal LRP4 directs the development, maturation, and cytoskeletal organization of peripheral synapses.
DePew AT, Bruckner JJ, O'Connor-Giles KM, Mosca TJ. DePew AT, et al. bioRxiv [Preprint]. 2023 Nov 8:2023.11.03.564481. doi: 10.1101/2023.11.03.564481. bioRxiv. 2023. PMID: 37961323 Free PMC article. Updated. Preprint.
References
- Bourouis M (2002) Targeted increase in shaggy activity levels blocks wingless signaling. Genesis 34: 99-102. - PubMed
- Broadie KS, Richmond JE (2002) Establishing and sculpting the synapse in Drosophila and C. elegans Curr Opin Neurobiol 12: 491-498. - PubMed
- Cohen P, Frame S (2001) The renaissance of GSK3. Nat Rev Mol Cell Biol 2: 769-776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases