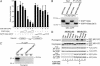CCAAT displacement protein/cut homolog recruits G9a histone lysine methyltransferase to repress transcription - PubMed (original) (raw)
CCAAT displacement protein/cut homolog recruits G9a histone lysine methyltransferase to repress transcription
Hitomi Nishio et al. Proc Natl Acad Sci U S A. 2004.
Abstract
CCAAT displacement protein/cut homolog (CDP/cut) is a highly conserved homeodomain protein that contains three cut repeat sequences. CDP/cut is a transcriptional factor for many diverse cellular and viral genes that are involved in most cellular processes, including differentiation, development, and proliferation. Here, we report that CDP/cut interacts with a histone lysine methyltransferase (HKMT), G9a, in vivo and in vitro. The deletion of the cut repeats within CDP/cut abrogates the interaction with G9a. The transcriptional repressor function of CDP/cut is mediated through HKMT activity of G9a associated with CDP/cut. We show that the recruitment of G9a to the human p21(waf1/cdi1) promoter is contingent on the interaction with CDP/cut, and CDP/cut is directly associated with an increase in the methylation in vivo of Lys-9 in histone H3 within the CDP/cut-regulatory region of the p21(waf1/cdi1) promoter. The endogenous level of p21(waf1/cdi1) expression is repressed through CDP/cut and mediated by HKMT activity of G9a. Furthermore, we report the identification of G9a as a component of CDP/cut complex. G9a colocalizes with CDP/cut in the nucleus. These results indicate that G9a functions as a transcriptional corepressor in association with a CDP/cut complex. These studies now reveal the interaction of G9a with a sequence-specific transcription factor that regulates gene repression through CDP/cut.
Figures
Fig. 1.
CDP/cut interacts with hG9a in vivo and in vitro.(A) hG9a coimmunoprecipitates with FLAG-tagged CDP/cut from cell extracts of transfected 293T cells. Cells were transfected with FLAG-CDP/cut and/or EGFP-hG9a expression vectors as indicated. Whole-cell extracts were immunoprecipitated with anti-FLAG Ab, and immunoprecipitates were analyzed by Western blot with anti-GFP Ab. (B) Schematic representation of CDP/cut with evolutionarily conserved domains (cc, coiled coil; cut, cut repeat; HD, homeodomain) and truncated derivatives. (C) Association with hG9a is disrupted by C-terminal truncation in CDP/cut. Immunoprecipitates from transfected cells with expression vectors for EGFP-hG9a and FLAG-CDP/cut truncated derivatives were analyzed as in A.(D) GST-CDPC (C-terminal region of CDP/cut) fusion protein interacts with _in vitro_-translated hG9a. GST pull-down assays were performed with _in vitro_-translated [35S]hG9a and GST fusion proteins as indicated. Input lane shows 10% of total.
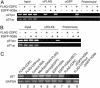
Fig. 2.
CDP/cut represses transcription through HKMT activity of hG9a. (A) hG9a corepresses transcription with CDP/cut. The 293T cells were transfected with a Gal4-driven luciferase reporter with combinations of the expression vector for Gal4DBD-CDPC and increasing amounts of the EGFP-hG9a or EGFP-hG9a (ΔSET) expression vector. Luciferase activity was measured 48 h after transfection. The means ± SD of duplicate determinations from five separate experiments are shown. (B) hG9a (ΔSET) interacts with CDPC. The 293T cells were transfected with FLAG-CDPC and EGFP-hG9a or EGFP-hG9a (ΔSET) expression vectors. Whole-cell extracts were immunoprecipitated with anti-FLAG Ab, and immunoprecipitates were analyzed by Western blot with anti-GFP Ab. (C) Immunoprecipitated EGFP-hG9a possesses histone H3 methyltransferase activity, but EGFP-hG9a (ΔSET) does not. The 293T cells were transfected with EGFP-hG9a or EGFP-hG9a (ΔSET) expression vector, and the whole-cell extracts were immunoprecipitated with anti-GFP Ab. Immunoprecipitates were assayed for in vitro HMT activity by using histone H3 and H4 as substrates. (D) CDP/_cut_-mediated repression is associated with H3-K9 and H3-K27 methyltransferase activity by G9a. The 293T cells were transfected, and the whole-cell extracts were immunoprecipitated as in B. Immunoprecipitates were assayed for in vitro HMT activity by using GST-histone H3 (amino acids 1–57) fusion proteins (WT and mutants) as substrates. Also shown are Coomassie blue staining of purified GST-histone H3 fusion proteins introduced into assays as well as Western blots with Abs against CDP and GFP of inputs and immunoprecipitates with anti-FLAG Ab.
Fig. 3.
CDP/cut recruits hG9a to repress p21_waf1/cdi1_. (A) hG9a recruitment to the p21pro is CDP/cut dependent. ChIPs from transfected 293T cells with combinations of FLAG-CDPC and EGFP-hG9a expression vectors as indicated were performed with anti-FLAG Ab, anti-GFP Ab, or normal serum. Immunoprecipitates were analyzed for the presence of the p21pro and the upstream region (p21up) by PCR amplification. Results are representative of three independent experiments. (B) hG9a recruitment elevates dimethylated H3-Lys-9 in the p21pro. ChIPs were performed as in A except that experiments were carried out with anti-dimethyl H3-Lys-9 Ab. (C) p21_waf1/cdi1_ expression is repressed by CDP/cut mediated through hG9a. Total RNA was isolated from human diploid fibroblast IMR90 cells that were transfected with expression vectors as indicated and analyzed for the expression of p21_waf1/cdi1_ by RT-PCR. Results are representative of three independent experiments.
Fig. 4.
hG9a is a component of CDP/cut complex. (A) Endogenous hG9a is a component of purified CDP/cut complex. (His)6-Xpress-CDPC complex was purified from 293 EBNA cells stably expressing (His)6-Xpress-CDPC by using Ni+-agarose, followed by immunoprecipitation with anti-Xpress Ab, and analyzed by SDS/PAGE and silver staining. Identities of protein bands were determined by MS. As a control, mock purification was performed from 293 EBNA cells generated with an empty (His)6-Xpress-tagged protein expression vector. (B) Endogenous hG9a specifically associates with the CDP/cut complex. The eluates of (His)6-Xpress-CDPC complex in A were analyzed by Western blots with Abs against CDP, TAFII100, Sp1, or G9a. (C) Endogenous hG9a associates with endogenous CDP/cut. The eluates of (His)6-Xpress-CDPC complex in A were immunoprecipitated with anti-CDP Ab, anti-G9a Ab, or normal serum, and the immunoprecipitates were analyzed by Western blot with anti-CDP Ab.
Fig. 5.
hG9a colocalizes with CDP/cut in the nucleus. The 293T cells were transfected with an expression vector for FLAG-hG9a and double-stained with anti-FLAG and anti-CDP Abs. FLAG-hG9a was detected with FITC-conjugated secondary Ab, and endogenous CDP/cut was detected with Rhodamine-conjugated secondary Ab. Representative red immunofluorescent image for endogenous CDP/cut (Left), green immunofluorescent image for FLAG-hG9a (Center), and merged image (Right) are shown. The yellow dots in the merged images indicate colocalizaion of hG9a and CDP/cut.
Similar articles
- Gfi1 coordinates epigenetic repression of p21Cip/WAF1 by recruitment of histone lysine methyltransferase G9a and histone deacetylase 1.
Duan Z, Zarebski A, Montoya-Durango D, Grimes HL, Horwitz M. Duan Z, et al. Mol Cell Biol. 2005 Dec;25(23):10338-51. doi: 10.1128/MCB.25.23.10338-10351.2005. Mol Cell Biol. 2005. PMID: 16287849 Free PMC article. - Regulation of the homeodomain CCAAT displacement/cut protein function by histone acetyltransferases p300/CREB-binding protein (CBP)-associated factor and CBP.
Li S, Aufiero B, Schiltz RL, Walsh MJ. Li S, et al. Proc Natl Acad Sci U S A. 2000 Jun 20;97(13):7166-71. doi: 10.1073/pnas.130028697. Proc Natl Acad Sci U S A. 2000. PMID: 10852958 Free PMC article. - Histone H3 lysine 9 methyltransferase G9a is a transcriptional coactivator for nuclear receptors.
Lee DY, Northrop JP, Kuo MH, Stallcup MR. Lee DY, et al. J Biol Chem. 2006 Mar 31;281(13):8476-85. doi: 10.1074/jbc.M511093200. Epub 2006 Feb 4. J Biol Chem. 2006. PMID: 16461774 Free PMC article. - G9a, a multipotent regulator of gene expression.
Shankar SR, Bahirvani AG, Rao VK, Bharathy N, Ow JR, Taneja R. Shankar SR, et al. Epigenetics. 2013 Jan;8(1):16-22. doi: 10.4161/epi.23331. Epub 2012 Dec 20. Epigenetics. 2013. PMID: 23257913 Free PMC article. Review.
Cited by
- G9a is essential for EMT-mediated metastasis and maintenance of cancer stem cell-like characters in head and neck squamous cell carcinoma.
Liu S, Ye D, Guo W, Yu W, He Y, Hu J, Wang Y, Zhang L, Liao Y, Song H, Zhong S, Xu D, Yin H, Sun B, Wang X, Liu J, Wu Y, Zhou BP, Zhang Z, Deng J. Liu S, et al. Oncotarget. 2015 Mar 30;6(9):6887-901. doi: 10.18632/oncotarget.3159. Oncotarget. 2015. PMID: 25749385 Free PMC article. - Functional dynamics of H3K9 methylation during meiotic prophase progression.
Tachibana M, Nozaki M, Takeda N, Shinkai Y. Tachibana M, et al. EMBO J. 2007 Jul 25;26(14):3346-59. doi: 10.1038/sj.emboj.7601767. Epub 2007 Jun 28. EMBO J. 2007. PMID: 17599069 Free PMC article. - The roles of CUX1 homeodomain proteins in the establishment of a transcriptional program required for cell migration and invasion.
Kedinger V, Nepveu A. Kedinger V, et al. Cell Adh Migr. 2010 Jul-Sep;4(3):348-52. doi: 10.4161/cam.4.3.11407. Epub 2010 Jul 4. Cell Adh Migr. 2010. PMID: 20224295 Free PMC article. - IFITM1 suppression blocks proliferation and invasion of aromatase inhibitor-resistant breast cancer in vivo by JAK/STAT-mediated induction of p21.
Lui AJ, Geanes ES, Ogony J, Behbod F, Marquess J, Valdez K, Jewell W, Tawfik O, Lewis-Wambi J. Lui AJ, et al. Cancer Lett. 2017 Jul 28;399:29-43. doi: 10.1016/j.canlet.2017.04.005. Epub 2017 Apr 12. Cancer Lett. 2017. PMID: 28411130 Free PMC article. - Identification of three histone methyltransferases in Drosophila: dG9a is a suppressor of PEV and is required for gene silencing.
Mis J, Ner SS, Grigliatti TA. Mis J, et al. Mol Genet Genomics. 2006 Jun;275(6):513-26. doi: 10.1007/s00438-006-0116-x. Epub 2006 Apr 19. Mol Genet Genomics. 2006. PMID: 16622709
References
- Skalnik, D. G., Strauss, E. C. & Orkin, S. H. (1991) J. Biol. Chem. 266, 16736–16744. - PubMed
- van Wijnen, A. J., Cooper, C., Odgren, P., Aziz, F., De Luca, A., Shakoori, R. A., Giordano, A., Quesenberry, P. J., Lian, J. B., Stein, G. S., et al. (1997) J. Cell. Biochem. 66, 512–523. - PubMed
- van Gurp, M. F., Pratap, J., Luong, M., Javed, A., Hoffmann, H., Giordano, A., Stein, J. L., Neufeld, E. J., Lian, J. B., Stein, G. S., et al. (1999) Cancer Res. 59, 5980–5988. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials