A STAT4-dependent Th1 response is required for resistance to the helminth parasite Taenia crassiceps - PubMed (original) (raw)
A STAT4-dependent Th1 response is required for resistance to the helminth parasite Taenia crassiceps
Miriam Rodríguez-Sosa et al. Infect Immun. 2004 Aug.
Abstract
To determine the role of STAT4-dependent Th1 responses in the regulation of immunity to the helminth parasite Taenia crassiceps, we monitored infections with this parasite in resistant mice lacking the STAT4 gene. While T. crassiceps-infected STAT4(+/+) mice rapidly resolved the infection, STAT4(-/-) mice were highly susceptible to infection and displayed large parasite loads. Moreover, the inability of STAT4(-/-) mice to control the infection was associated with the induction of an antigen-specific Th2-type response characterized by significantly higher levels of Th2-associated immunoglobulin G1 (IgG1) and total IgE as well as interleukin-4 (IL-4), IL-10, and IL-13 than those in STAT4(+/+) mice, who produced significantly more gamma interferon. Furthermore, early after infection, macrophages from STAT4(-/-) mice produced lower levels of the pro-inflammatory cytokines IL-12, tumor necrosis factor alpha, IL-1 beta, and nitric oxide (NO) than those from STAT4(+/+) mice, suggesting a pivotal role for macrophages in mediating protection against cysticercosis. These findings demonstrate a critical role for the STAT4 signaling pathway in the development of a Th1-type immune response that is essential for mediating protection against the larval stage of T. crassiceps infection.
Figures
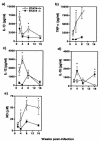
FIG. 1.
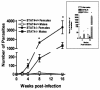
STAT4−/− mice are highly susceptible while STAT4+/+ mice are highly resistant to T. crassiceps infection. The graph shows the course of T. crassiceps infection in STAT4−/− and STAT4+/+ mice after i.p. infection with 20 cysticerci. Data are expressed as mean parasite loads ± standard errors (SE) of six mice per group. *, P < 0.01 for STAT4−/− versus STAT4+/+ data at the same time point. The data shown are representative of two independent experiments. Inset, C57BL/6 and STAT4+/+ littermates displayed similar resistance patterns, while partially backcrossed STAT4−/− mice were highly susceptible to T. crassiceps infection. *, P < 0.05 for STAT4−/− versus STAT4+/+ and C57BL/6 mice (five to six mice per group).
FIG. 2.
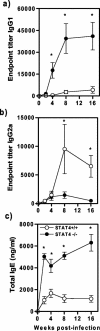
Kinetics of antibody production during T. crassiceps infection of STAT4−/− (closed circles) and STAT4+/+ (open circles) mice. (a) Anti-T. crassiceps IgG1. (b) Anti-T. crassiceps IgG2a. (c) Total IgE. The graphs show mean titers ± SE (n = 8 animals) and are representative of two independent experiments. *, P < 0.05 for STAT4−/− versus STAT4+/+ data at the same time point.
FIG. 3.
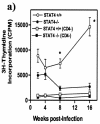
Kinetics of in vitro cytokine production by TcAg-stimulated spleen cells from STAT4−/− and STAT4+/+ mice. (a) IFN-γ; (b) IL-10; (c) IL-4; (d) IL-13. The graphs show cytokine production by splenocytes after 72 h of in vitro stimulation with TcAg (50 μg/ml).
FIG. 4.
Cell type analysis of proliferative responses to TcAg-specific stimulation. (a) Splenocytes from STAT4−/− and wild-type mice taken 2, 4, 8, and 16 weeks after T. crassiceps infection were stimulated with 50 μg of TcAg/ml. In some experiments, CD4 cells were magnetically removed and CD4− splenocytes were stimulated in the same way as total splenocytes. *, P < 0.05 for STAT4−/− versus STAT4+/+ data at the same time points. (b) TcAg-specific proliferation of CD4 and CD8 cells. Spleen cells from infected wild-type and STAT4−/− mice (at 2 and 16 weeks postinfection) were labeled with CFSE and cultured with 50 μg of TcAg/ml for 4 days prior to staining with a PE-conjugated anti-CD4 or anti-CD8 monoclonal antibody and analysis on a flow cytometer. Five thousand nonproliferating, CFSE+ (CD4+ or CD8+) cells were collected, and the percentages of proliferating and nonproliferating cells were calculated and are indicated in each dot plot. The results shown are representative of those obtained for three mice per group.
FIG. 4.
Cell type analysis of proliferative responses to TcAg-specific stimulation. (a) Splenocytes from STAT4−/− and wild-type mice taken 2, 4, 8, and 16 weeks after T. crassiceps infection were stimulated with 50 μg of TcAg/ml. In some experiments, CD4 cells were magnetically removed and CD4− splenocytes were stimulated in the same way as total splenocytes. *, P < 0.05 for STAT4−/− versus STAT4+/+ data at the same time points. (b) TcAg-specific proliferation of CD4 and CD8 cells. Spleen cells from infected wild-type and STAT4−/− mice (at 2 and 16 weeks postinfection) were labeled with CFSE and cultured with 50 μg of TcAg/ml for 4 days prior to staining with a PE-conjugated anti-CD4 or anti-CD8 monoclonal antibody and analysis on a flow cytometer. Five thousand nonproliferating, CFSE+ (CD4+ or CD8+) cells were collected, and the percentages of proliferating and nonproliferating cells were calculated and are indicated in each dot plot. The results shown are representative of those obtained for three mice per group.
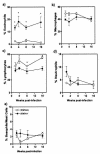
FIG. 5.
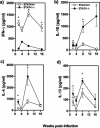
Peritoneal macrophages from STAT4−/− (closed circles) and STAT4+/+ (open circles) _T. crassiceps_-infected mice display different responses after in vitro stimulation with LPS (1 μg/ml) plus IFN-γ (2 ng/ml) for 24 h. IL-1β (a), TNF-α (b), IL-12 (c), IL-18 (d), and NO (e) production by macrophages at different weeks after T. crassiceps infection was determined by ELISA or the Griess reaction. Data are the means ± standard deviations for six animals at each time point. *, P < 0.05 for STAT4−/− versus STAT4+/+ data at the same time points.
FIG. 6.
T. crassiceps infection recruits significantly larger numbers of eosinophils to the site of infection in STAT4−/− mice. (a) Percentages of eosinophils; (b) percentages of macrophages; (c) percentages of lymphocytes; (d) percentages of neutrophils; (e) percentages of basophils/mast cells. The graphs show the recruitment of cells to the peritoneal cavity after T. crassiceps infection. Mice were infected i.p. with 20 cysticerci and were sacrificed at the indicated times. The composition of cells was analyzed after a cytospin and Wright-Giemsa staining, with at least 300 cells counted per slide. Analyses were performed in individual mice (six mice per group). The data are representative of two independent experiments. *, P < 0.05 for STAT4−/− versus STAT4+/+ data at the same time points.
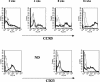
FIG. 7.
T. crassiceps infection up-regulates the expression of CD23 and CCR5 in peritoneal macrophages from STAT4−/− mice. Peritoneal macrophages from STAT4+/+ and STAT4−/− infected mice were obtained at different time points after infection and stained with anti-F4/80 in combination with anti-CD23-PE, anti-CCR5-PE, or an isotype control antibody. The histograms shown were gated on the F4/80+ adherent peritoneal cells (dotted line, isotype; thick line, STAT4−/− mice; dashed line, STAT4+/+ mice). The data are representative of two independent experiments, with three to four mice per group.
Similar articles
- Nitric oxide contributes to host resistance against experimental Taenia crassiceps cysticercosis.
Alonso-Trujillo J, Rivera-Montoya I, Rodríguez-Sosa M, Terrazas LI. Alonso-Trujillo J, et al. Parasitol Res. 2007 May;100(6):1341-50. doi: 10.1007/s00436-006-0424-4. Epub 2007 Jan 6. Parasitol Res. 2007. PMID: 17206501 - Cutting edge: susceptibility to the larval stage of the helminth parasite Taenia crassiceps is mediated by Th2 response induced via STAT6 signaling.
Rodriguez-Sosa M, David JR, Bojalil R, Satoskar AR, Terrazas LI. Rodriguez-Sosa M, et al. J Immunol. 2002 Apr 1;168(7):3135-9. doi: 10.4049/jimmunol.168.7.3135. J Immunol. 2002. PMID: 11907063 - Stat4- and Stat6-deficient mice as models for manipulating T helper cell responses.
Grusby MJ. Grusby MJ. Biochem Soc Trans. 1997 May;25(2):359-60. doi: 10.1042/bst0250359. Biochem Soc Trans. 1997. PMID: 9191117 Review. No abstract available. - The neuroimmunoendocrine network in the complex host-parasite relationship during murine cysticercosis.
Morales-Montor J, Escobedo G, Vargas-Villavicencio JA, Larralde C. Morales-Montor J, et al. Curr Top Med Chem. 2008;8(5):400-7. doi: 10.2174/156802608783790866. Curr Top Med Chem. 2008. PMID: 18393903 Review.
Cited by
- Similarity and diversity in macrophage activation by nematodes, trematodes, and cestodes.
Jenkins SJ, Allen JE. Jenkins SJ, et al. J Biomed Biotechnol. 2010;2010:262609. doi: 10.1155/2010/262609. Epub 2010 Jan 26. J Biomed Biotechnol. 2010. PMID: 20145705 Free PMC article. Review. - In vivo albendazole treatment of Taenia crassiceps cysticerci strain WFU: proliferation, damage, and recovery.
Zurabian R, Aguilar-Vega L, Terrones Vargas E, Cervera Hernández ME, Willms K, Ruíz-Velasco Acosta S. Zurabian R, et al. Parasitol Res. 2013 Nov;112(11):3961-8. doi: 10.1007/s00436-013-3589-7. Epub 2013 Sep 5. Parasitol Res. 2013. PMID: 24005476 - Diversity and dialogue in immunity to helminths.
Allen JE, Maizels RM. Allen JE, et al. Nat Rev Immunol. 2011 Jun;11(6):375-88. doi: 10.1038/nri2992. Nat Rev Immunol. 2011. PMID: 21610741 Review. - Nitric oxide contributes to host resistance against experimental Taenia crassiceps cysticercosis.
Alonso-Trujillo J, Rivera-Montoya I, Rodríguez-Sosa M, Terrazas LI. Alonso-Trujillo J, et al. Parasitol Res. 2007 May;100(6):1341-50. doi: 10.1007/s00436-006-0424-4. Epub 2007 Jan 6. Parasitol Res. 2007. PMID: 17206501 - E-cadherin expression in macrophages dampens their inflammatory responsiveness in vitro, but does not modulate M2-regulated pathologies in vivo.
Van den Bossche J, Laoui D, Naessens T, Smits HH, Hokke CH, Stijlemans B, Grooten J, De Baetselier P, Van Ginderachter JA. Van den Bossche J, et al. Sci Rep. 2015 Jul 31;5:12599. doi: 10.1038/srep12599. Sci Rep. 2015. PMID: 26226941 Free PMC article.
References
- Allen, J. E., R. A. Lawrence, and R. M. Maizels. 1996. APC from mice harbouring the filarial nematode, Brugia malayi, prevent cellular proliferation but not cytokine production. Int. Immunol. 8:143-151. - PubMed
- Allen, J. E., and R. M. Maizels. 1997. Th1-Th2: reliable paradigm or dangerous dogma? Immunol. Today 18:387-392. - PubMed
- Anderson, S., V. L. Shires, R. A. Wilson, and A. P. Mountford. 1998. In the absence of IL-12, the induction of Th1-mediated protective immunity by the attenuated schistosome vaccine is impaired, revealing an alternative pathway with Th2-type characteristics. Eur. J. Immunol. 28:2827-2838. - PubMed
- Bancroft, A. J., D. Artis, D. D. Donaldson, J. P. Sypek, and R. K. Grencis. 2000. Gastrointestinal nematode expulsion in IL-4 knockout mice is IL-13 dependent. Eur. J. Immunol. 30:2083-2091. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous