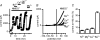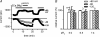Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: permeation of divalent cations and pH-mediated regulation - PubMed (original) (raw)
Comparative Study
Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: permeation of divalent cations and pH-mediated regulation
Asfree Gwanyanya et al. J Physiol. 2004.
Abstract
Cardiac tissue expresses several TRP proteins as well as a Mg2+ -inhibited, non-selective cation current (IMIC) that bears many characteristics of TRP channel currents. We used the whole-cell voltage clamp technique in pig and rat ventricular myocytes to characterize the permeation, blockage properties and regulation of the cardiac IMIC channels in order to compare them with TRP channels, in particular with Mg2+ -sensitive TRPM6 and TRPM7. We show that removing extracellular divalent cations unmasks large inward and outward monovalent currents, which can be inhibited by intracellular Mg2+. Inward currents are suppressed upon replacing extracellular Na+ by NMDG+. Divalent cations block monovalent IMIC and, at 10-20 mm, carry measurable currents. Their efficacy sequence in decreasing outward IMIC (Ni2+ = Mg2+ > Ca2+ > Ba2+) and in inducing inward IMIC (Ni2+ >> Mg2+ = Ca2+ approximately Ba2+), and their permeabilities calculated from reversal potentials are similar to those of TRPM6 and TRPM7 channels. The trivalent cations Gd3+ and Dy3+ also block IMIC in a voltage-dependent manner (delta = 0.4-0.5). In addition they inhibit the inward current carried by divalent cations. IMIC is regulated by pH. Decreasing or increasing extracellular pH decreased and increased IMIC, respectively (pH0.5 = 6.9, nH = 0.98). Qualitatively similar results were obtained on IMIC in rat basophilic leukaemia cells. These effects in cardiac myocytes were absent in the presence of high intracellular buffering by 40 mm Hepes. Our results suggest that IMIC in cardiac cells is due to TRPM channels, most probably to TRPM6 or TRPM7 channels or to their heteromultimeres.
Figures
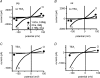
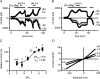
Figure 1. Removal of extracellular divalent cations (Cao2+, Mgo2+,) unmasks large outward and inward monovalent cation current (_I_MIC) in myocytes of pig (A and C) and rat (B and D)
Original traces of currents obtained using voltage ramps in the presence of divalent cations (1.8 m
m
Ca2+o, 0.9 Mg2+o; •), during their depletion (nominally 0 m
m
Ca2+o and 0 m
m
Mg2+o; ^) and after their re-addition (▪). Internal free [Mg2+]i: 20 μ
m
. A and B, cells dialysed without TEA+. No inward-going rectification of _I_MIC in the absence of internal TEA+. C and D, cells dialysed with internal solution containing 25 m
m
TEA+. Notice a flattening of the current–voltage relationship of _I_MIC (causing apparent inward-going rectification).
Figure 2. Accounting for the effect of removing extracellular divalent cations by one single membrane conductance
A,C and E, time course of changes in membrane currents during extracellular divalent cation depletion and re-addition. Periods of exposure to solutions of divalent cation-free solution are indicated by horizontal bars. B, D and F, MIC current, obtained as difference between traces in the absence and in the presence of Ca2+o and Mg2+o. Currents were taken at different times, and their labels indicate the corresponding sampling times in panels A, C and E. Notice constancy of reversal potential during different time courses of inward and outward currents, and during run-down. Internal free [Mg2+]i: 20 μ
m
in A and C, 0 m
m
in E. External solution change with bath perfusion in A and C, with fast perfusion system in E.
Figure 3. Voltage-dependent block of MIC current by divalent cations
A, time course of changes in membrane currents at −120, −80 and +80 mV during extracellular divalent cation depletion, and following the addition of 10 μ
m
or 20 μ
m
Ca2+. Currents measured using voltage ramps. Periods of exposure to divalent cation-free solutions and to 10 μ
m
or 20 μ
m
Ca2+ are indicated by horizontal bars. B, current–voltage relationships in control conditions (in the presence of extracellular divalent cations; ▪), during extracellular divalent cation depletion (^), and following the the addition of 10 μ
m
(•) or 20 μ
m
Ca2+ (□). C, MIC current–voltage relationship. _I_MIC obtained as difference current between traces in the nominal absence of divalent cations and in the presence of Ca2+. Reference _I_MIC in nominal absence of Ca2+o (^) is partly blocked by 10 μ
m
(•) or 20 μ
m
Ca2+ (□). Notice marked block at negative but not at positive potentials. D, relative _I_MIC remaining in the presence of 10 μ
m
or 20 μ
m
Ca2+. Values near reversal potential were removed because of large scatter due to divisions by small numbers. Notice also relief from block at extreme negative potentials. Line through data points drawn by fitting with of eqn (A5) Appendix. Internal free [Mg2+]i: 20 μ
m
. Extracellular solution change with bath perfusion. Rat ventricular myocyte.
Figure 4. Voltage-dependent block of MIC current by trivalent cations
A and B, time course of changes in membrane currents at −120, −80 and +80 mV during extracellular divalent cation depletion, in the absence and in the presence of either 10 μ
m
(A) or 100 μ
m
Gd3+ (B). Currents measured using voltage ramps. Periods of exposure to divalent cation-free solutions and to trivalent cations are indicated by horizontal bars. Phosphate included in the divalent cation-free solution in B but not A during wash-out of Gd3+. Notice marked block caused by Gd3+ at negative but not at positive potentials. C and D, relative _I_MIC remaining in the presence of the trivalent ions Gd3+ (C) or Dy3+ (D). Values near reversal potential were removed because of large scatter due to divisions by small numbers. Notice also incomplete block at extreme negative potentials. Line through data points drawn by fitting with of eqn (A5) Appendix. Internal free [Mg2+]i: 20 μ
m
. Extracellular solution change with either fast perfusion system (A) or bath perfusion (B). Slow onset of Gd3+-induced block in B is probably related to the slow concentration change during bath solution change. Pig (A–C) and rat (D) ventricular myocytes.
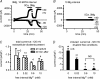
Figure 5. Spontaneous run-up of _I_MIC during wash-out of internal Mg2+, but absence of measurable divalent cation entry with physiological extracellular divalent cation concentrations
A and B, time evolution of currents measured at −120, −80 and +80 mV using voltage ramps. Periods of exposure to solution without Ca2+o and Mg2+o indicated by horizontal bars. C, effect of internal Mg2+ on the total inward current at −120 mV in the presence of extracellular divalent cations. Currents (means ±
s.e.m.
) were measured at the beginning of patch rupture and after 20 min. Internal free Mg2+ concentration calculated using CaBuf (see Methods). Difference between values in the various columns were not significant (paired t test used for comparing data of same [Mg2+]i at the beginning of patch rupture versus after 20 min; ANOVA used for all data of different [Mg2+]i). D, effect of internal Mg2+ on the current induced at −120 mV upon removal of the divalent cations. *P < 0.001 when comparing (by ANOVA with post hoc t test) with data at 20 μ
m
free [Mg2+]i. NS, non-significant difference (P > 0.05). Extracellular solution change with fast perfusion system. Pig ventricular myocytes. Notice (1) run-up of outward current but absence of significant increase of inward current during prolonged dialysis with 1.8 m
m
Ca2+o and 0.9 m
m
Mg2+o, in the external solution, and (2) suppression of _I_MIC by internal Mg2+.
Figure 6. Block of outward currents by divalent cations
A, time course of changes in membrane currents in Na+-free extracellular solution (Na+ replaced by NMDG+), in the absence of extracellular divalent cations and during addition of various divalent cations. Currents measured at +80 mV. Periods of exposure to the divalent cations are indicated by horizontal bars. B, original traces of currents obtained using voltage ramps in the various conditions: in NMDG+ in the absence of divalent cations, and during addition of 10 m
m
Ni2+o, Mg2+o, Ca2+o or Ba2+o. C, means and
s.e.m.
of the outward current (expressed as percentage relative to the current in NMDG+ alone) remaining in the presence of various divalent cations. Internal free [Mg2+]i: 0 m
m
. Extracellular solution change with fast perfusion system. Pig ventricular myocytes.
Figure 7. Permeability to divalent cations
A and C, time course of changes in membrane currents in Na+-free extracellular solution (Na+ replaced by NMDG+), in the absence of extracellular divalent cations and during addition of various divalent cations at 20 m
m
. Currents measured at −120 mV. Periods of exposure to the divalent cations are indicated by horizontal bars. In C, 1 m
m
Gd3+ was added in the presence of 20 m
m
Ni2+. B, means and
s.e.m.
of the inward current density (in pA pF−1) induced upon addition of the various divalent cations. Inset: current–voltage relations. D, original traces of currents obtained using voltage ramps in the various conditions: in NMDG+ in the absence of divalent cations (^), in the presence of 20 m
m
Ni2+ (•), and in the presence of 1 m
m
Gd3+ added in addition to Ni2+ (▪). Internal free [Mg2+]i: 0 m
m
. Extracellular solution change with fast perfusion system. Pig ventricular myocytes.
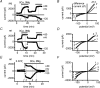
Figure 8. Regulation by pH
A and B, time course of changes in membrane currents during extracellular divalent cation depletion and repletion. Extracellular acidosis and alkalosis were applied in divalent-free solutions. C, relative divalent-sensitive current at different pH values. Current at pH 7.4 taken as 100%. Currents were measured at +80 mV (▪), −80 mV (^) and −120 mV (•). D, current–voltage relationships of _I_MIC at different pH values. Internal free [Mg2+]i: 20 μ
m
. Extracellular solution change with bath perfusion. Pig ventricular myocytes.
Figure 9. High intracellular pH buffering prevents changes of _I_MIC upon extracellular acidification
A, time course of changes in membrane currents during extracellular divalent cation depletion and repletion. Extracellular acidosis was applied in divalent-free solutions. Cell dialysed with pipette solution containing 40 m
m
Hepes measured at +80, −80 and −120 mV. Periods of exposure to divalent cation-free and acidic solutions are indicated by horizontal bars. B, mean values of relative _I_MIC (divalent cation-sensitive current) in cells dialysed with high Hepes at normal and acidic pH values. Current at pH 7.4 taken as 100%. Internal free [Mg2+]i: 20 μ
m
. Extracellular solution change with fast perfusion system. Pig ventricular myocytes.
Figure 10. MIC current in rat basophilic leukaemia cells (RBL) and its regulation by pH
A and B, unmasking of _I_MIC by the removal of extracellular divalent cations (Ca2+o, Mg2+o). Original traces of currents obtained using voltage ramps in the presence of divalent cations (1.8 m
m
Ca2+o, 0.9 Mg2+o; •) and during their depletion (nominally 0 m
m
Ca2+o and 0 m
m
Mg2+o; ^). A, cell dialysed without TEA+; no inward-going rectification of _I_MIC. B, cell dialysed with internal solution containing 25 m
m
TEA+. Notice _I_MIC apparent inward-going rectification. C, time course of changes in membrane currents measured at −90 mV during extracellular divalent cation depletion and re-addition. Extracellular pH was changed while superfusing with the divalent-free solutions. D, pHo dependence of MIC current. Relative divalent-sensitive current (with current at pH 7.4 taken as 100%) at different pH values. Internal free [Mg2+]i: 0 m
m
. Extracellular solution change with fast perfusion system.
Similar articles
- Extracellular divalent cations block a cation non-selective conductance unrelated to calcium channels in rat cardiac muscle.
Mubagwa K, Stengl M, Flameng W. Mubagwa K, et al. J Physiol. 1997 Jul 15;502 ( Pt 2)(Pt 2):235-47. doi: 10.1111/j.1469-7793.1997.235bk.x. J Physiol. 1997. PMID: 9263906 Free PMC article. - Modulation of Human Cardiac TRPM7 Current by Extracellular Acidic pH Depends upon Extracellular Concentrations of Divalent Cations.
Mačianskienė R, Almanaitytė M, Jekabsone A, Mubagwa K. Mačianskienė R, et al. PLoS One. 2017 Jan 27;12(1):e0170923. doi: 10.1371/journal.pone.0170923. eCollection 2017. PLoS One. 2017. PMID: 28129376 Free PMC article. - Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7.
Li M, Du J, Jiang J, Ratzan W, Su LT, Runnels LW, Yue L. Li M, et al. J Biol Chem. 2007 Aug 31;282(35):25817-30. doi: 10.1074/jbc.M608972200. Epub 2007 Jun 28. J Biol Chem. 2007. PMID: 17599911 Free PMC article. - The Mg2+ and Mg(2+)-nucleotide-regulated channel-kinase TRPM7.
Penner R, Fleig A. Penner R, et al. Handb Exp Pharmacol. 2007;(179):313-28. doi: 10.1007/978-3-540-34891-7_19. Handb Exp Pharmacol. 2007. PMID: 17217066 Free PMC article. Review. - Role of kinase-coupled TRP channels in mineral homeostasis.
Chubanov V, Mittermeier L, Gudermann T. Chubanov V, et al. Pharmacol Ther. 2018 Apr;184:159-176. doi: 10.1016/j.pharmthera.2017.11.003. Epub 2017 Nov 9. Pharmacol Ther. 2018. PMID: 29129644 Review.
Cited by
- Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels.
Kozak JA, Matsushita M, Nairn AC, Cahalan MD. Kozak JA, et al. J Gen Physiol. 2005 Nov;126(5):499-514. doi: 10.1085/jgp.200509324. J Gen Physiol. 2005. PMID: 16260839 Free PMC article. - The Drosophila Trpm channel mediates calcium influx during egg activation.
Hu Q, Wolfner MF. Hu Q, et al. Proc Natl Acad Sci U S A. 2019 Sep 17;116(38):18994-19000. doi: 10.1073/pnas.1906967116. Epub 2019 Aug 19. Proc Natl Acad Sci U S A. 2019. PMID: 31427540 Free PMC article. - Importance of melastatin-like transient receptor potential 7 and cations (magnesium, calcium) in human osteoblast-like cell proliferation.
Abed E, Moreau R. Abed E, et al. Cell Prolif. 2007 Dec;40(6):849-65. doi: 10.1111/j.1365-2184.2007.00476.x. Cell Prolif. 2007. PMID: 18021175 Free PMC article. - Ca2+ Signalling and Hypoxia/Acidic Tumour Microenvironment Interplay in Tumour Progression.
Audero MM, Prevarskaya N, Fiorio Pla A. Audero MM, et al. Int J Mol Sci. 2022 Jul 2;23(13):7377. doi: 10.3390/ijms23137377. Int J Mol Sci. 2022. PMID: 35806388 Free PMC article. Review. - Overexpression of microRNA-202-3p protects against myocardial ischemia-reperfusion injury through activation of TGF-β1/Smads signaling pathway by targeting TRPM6.
Wu HY, Wu JL, Ni ZL. Wu HY, et al. Cell Cycle. 2019 Mar;18(5):621-637. doi: 10.1080/15384101.2019.1580494. Epub 2019 Feb 27. Cell Cycle. 2019. PMID: 30810438 Free PMC article. Retracted.
References
- Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. - PubMed
- Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. Am J Physiol Cell Physiol. 2000;278:C163–173. - PubMed
- Bakowski D, Parekh AB. Monovalent cation permeability and Ca2+ block of the store-operated Ca2+ current ICRAC in rat basophilic leukemia cells. Pflugers Arch. 2002;443:892–902. - PubMed
- Bosteels S, Matejovic P, Flameng W, Mubagwa K. Sodium influx via a non-selective pathway activated by the removal of extracellular divalent cations: possible role in the calcium paradox. Cardiovasc Res. 1999;43:417–425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous