Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer - PubMed (original) (raw)
Comparative Study
. 2004 Aug;240(2):306-12.
doi: 10.1097/01.sla.0000133355.48672.22.
Guenther Bayer, Klaus Aumayr, Susanne Taucher, Silvana Geleff, Margaretha Rudas, Ernst Kubista, Hubert Hausmaninger, Hellmut Samonigg, Michael Gnant, Raimund Jakesz, Reinhard Horvat; Austrian Breast and Colorectal Cancer Study Group
Affiliations
- PMID: 15273556
- PMCID: PMC1356408
- DOI: 10.1097/01.sla.0000133355.48672.22
Comparative Study
Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer
Sebastian F Schoppmann et al. Ann Surg. 2004 Aug.
Abstract
Objective: The aim of this study was to investigate the prognostic relevance of lymphangiogenesis and lymphovascular invasion in a large cohort of breast cancer patients.
Introduction: Invasion of tumor cells into blood and lymphatic vessels is one of the critical steps for metastasis. The presence or absence of lymph node metastasis is one of the main decision criteria for further therapy. One shortcoming of previous morphologic studies was the lack of specific markers that could exact discriminate between blood and lymphatic vessels. The aim of this study was to evaluate the prognostic relevance of lymphangiogenesis and lymphovascular invasion in breast cancer patients.
Methods: We investigated 374 tissue specimens of patients suffering from invasive breast cancer by immunostaining for the lymphatic endothelial specific marker podoplanin. Lymphangiogenesis, quantified by evaluating the lymphatic microvessels density (LMVD), and lymphovascular invasion (LVI) were correlated with various clinical parameters and prognostic relevance.
Results: LMVD correlated significantly with LVI (P = 0.001). LVI was associated significantly with a higher risk for developing lymph-node metastasis (P = 0.004). Calculating the prognostic relevance, LVI presented as an independent prognostic parameter for disease free as well as overall survival (P = 0.001, and P = 0.001, respectively).
Conclusion: Our data provide evidence that the biologic system of lymphangiogenesis constitutes a potential new target for development of anti-breast cancer therapeutic concepts. Our results further suggest that young, premenopausal patients with low differentiated breast tumors and high LMVD and LVI would, in particular, benefit from lymphangiogenesis-associated therapeutic strategies.
Figures
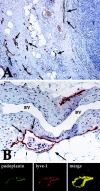
FIGURE 1. A: Breast cancer specimen with a high peritumoral LMVD (some of the lymphatic vessels stained for podoplanin are marked with arrows). Note the absence of lymphatic vessels in the tumor (T) (immunoperoxidase, original magnification ×200). B: Podoplanin-stained lymphatic vessel (arrows) with tumor cells (T) inside (LVI). Note the absence of endothelial podoplanin staining in a venous blood vessel (BV) with typical smooth muscle cells within its wall (immunoperoxidase, original magnification ×400). Line below shows immunofluorescence on lymphatic vessel for podoplanin and lyve-1, revealing perfect overlap (merge). Immunofluorescence, nuclear counterstaining with propidium iodide.
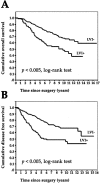
FIGURE 2. A: Overall survival in 374 breast cancer patients with (LVI**+) or without (LVI-) lymphovascular invasion. B: Disease-free survival in 374 breast cancer patients with (LVI+) or without (LVI-**) lymphovascular invasion.
Similar articles
- Lymphangiogenesis and lymphovascular invasion diminishes prognosis in esophageal cancer.
Schoppmann SF, Jesch B, Zacherl J, Riegler MF, Friedrich J, Birner P. Schoppmann SF, et al. Surgery. 2013 Apr;153(4):526-34. doi: 10.1016/j.surg.2012.10.007. Epub 2013 Jan 4. Surgery. 2013. PMID: 23294880 - VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival.
Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, Gnant M, Horvat R, Jakesz R, Birner P. Schoppmann SF, et al. Surgery. 2006 Jun;139(6):839-46. doi: 10.1016/j.surg.2005.12.008. Surgery. 2006. PMID: 16782443 - Hypoxia inducible factor-1alpha correlates with VEGF-C expression and lymphangiogenesis in breast cancer.
Schoppmann SF, Fenzl A, Schindl M, Bachleitner-Hofmann T, Nagy K, Gnant M, Horvat R, Jakesz R, Birner P. Schoppmann SF, et al. Breast Cancer Res Treat. 2006 Sep;99(2):135-41. doi: 10.1007/s10549-006-9190-3. Epub 2006 Mar 23. Breast Cancer Res Treat. 2006. PMID: 16555123 - The relationship of lymphatic vessel density, lymphovascular invasion, and lymph node metastasis in breast cancer: a systematic review and meta-analysis.
Zhang S, Zhang D, Yi S, Gong M, Lu C, Cai Y, Tang X, Zou L. Zhang S, et al. Oncotarget. 2017 Jan 10;8(2):2863-2873. doi: 10.18632/oncotarget.13752. Oncotarget. 2017. PMID: 27926511 Free PMC article. Review. - A meta-analysis of the relationship between lymphatic microvessel density and clinicopathological parameters in breast cancer.
Chen Y, Yan J, Yuan Z, Yu S, Yang C, Wang Z, Zheng Q. Chen Y, et al. Bull Cancer. 2013 Mar;100(3):1-10. doi: 10.1684/bdc.2013.1719. Bull Cancer. 2013. PMID: 23501839 Review.
Cited by
- Lymphovascular invasion and histologic grade are associated with specific genomic profiles in invasive carcinomas of the breast.
Fidalgo F, Rodrigues TC, Pinilla M, Silva AG, Maciel Mdo S, Rosenberg C, de Andrade VP, Carraro DM, Krepischi AC. Fidalgo F, et al. Tumour Biol. 2015 Mar;36(3):1835-48. doi: 10.1007/s13277-014-2786-z. Epub 2014 Nov 13. Tumour Biol. 2015. PMID: 25391423 Free PMC article. - Thrombocytes Correlate with Lymphangiogenesis in Human Esophageal Cancer and Mediate Growth of Lymphatic Endothelial Cells In Vitro.
Schoppmann SF, Alidzanovic L, Schultheis A, Perkmann T, Brostjan C, Birner P. Schoppmann SF, et al. PLoS One. 2013 Jun 26;8(6):e66941. doi: 10.1371/journal.pone.0066941. Print 2013. PLoS One. 2013. PMID: 23840559 Free PMC article. - Blood vessel invasion and other variables as predictors of long-term survival in Japanese and British patients with primary invasive breast cancer.
Kato T, Pezzella F, Steers G, Campo L, Leek RD, Turley H, Kameoka S, Nishikawa T, Harris AL, Gatter KC, Fox S. Kato T, et al. Int J Clin Exp Pathol. 2014 Oct 15;7(11):7967-78. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25550840 Free PMC article. - An overview of prognostic factors for long-term survivors of breast cancer.
Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW. Soerjomataram I, et al. Breast Cancer Res Treat. 2008 Feb;107(3):309-30. doi: 10.1007/s10549-007-9556-1. Epub 2007 Mar 22. Breast Cancer Res Treat. 2008. PMID: 17377838 Free PMC article. Review. - Lymph node ratio is more valuable than level III involvement for prediction of outcome in node-positive breast carcinoma patients.
Yildirim E, Berberoglu U. Yildirim E, et al. World J Surg. 2007 Feb;31(2):276-89. doi: 10.1007/s00268-006-0487-5. World J Surg. 2007. PMID: 17219275
References
- Donegan WL. Tumor-related prognostic factors for breast cancer. CA Cancer J Clin. 1997;47:28–51. - PubMed
- Fisher B, Bauer M, Wickerham DL, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer: an NSABP update. Cancer. 1983;52:1551–1557. - PubMed
- Nathanson SD, Zarbo RJ, Wachna DL, et al. Microvessels that predict axillary lymph node metastases in patients with breast cancer. Arch Surg. 2000;135:586–593; discussion 593–594. - PubMed
- Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. - PubMed
- Weidner N, Folkman J, Pozza F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical