Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma - PubMed (original) (raw)
Comparative Study
Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma
Xiao-Han Chen et al. Am J Pathol. 2004 Aug.
Abstract
Plaques composed of amyloid beta (Abeta) have been found within days following brain trauma in humans, similar to the hallmark plaque pathology of Alzheimer's disease (AD). Here, we evaluated the potential source of this Abeta and long-term mechanisms that could lead to its production. Inertial brain injury was induced in pigs via head rotational acceleration of 110 degrees over 20 ms in the coronal plane. Animals were euthanized at 3 hours, 3 days, 7 days, and 6 months post-injury. Immunohistochemistry and Western blot analyses of the brains were performed using antibodies specific for amyloid precursor protein (APP), Abeta peptides, beta-site APP-cleaving enzyme (BACE), presenilin-1 (PS-1), caspase-3, and caspase-mediated cleavage of APP (CCA). Substantial co-accumulation for all of these factors was found in swollen axons at all time points up to 6 months following injury. Western blot analysis of injured brains confirmed a substantial increase in the protein levels of these factors, particularly in the white matter. These data suggest that impaired axonal transport due to trauma induces long-term pathological co-accumulation of APP with BACE, PS-1, and activated caspase. The abnormal concentration of these factors may lead to APP proteolysis and Abeta formation within the axonal membrane compartment.
Figures
Figure 1
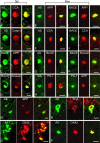
Representative photomicrographs demonstrating APP and NF protein accumulation in damaged axons in the subcortical white matter and basal ganglia detected by antibodies to 22C11/APP N-terminal and N52/NF200 at 3, 7 days, and 6 months following injury in the pig. Dark staining represents immunoreactivity of APP and NF proteins in swollen axons. Bar, 25 μm.
Figure 2
Representative photomicrographs revealing a modest number of pyknotic neurons in CA1 and CA3 subfields of the hippocampus (top panels) and foamy macrophage infiltrations within subcortical white matter, basal ganglia and lining blood vessels. Axonal bulbs were close to macrophage infiltrations (black arrows), detected by antibody OX42 at 7 days and 6 months post-injury (bottom panels). Bar, 25 μm.
Figure 3
Representative photomicrographs revealing Aβ accumulation in axonal bulbs in the basal ganglia at 3, 7 days, and 6 months after brain injury in the pig. Aβ was identified by antibodies to 4G8, BCO5, 6F/3D, Aβ1–40, 10Δ5. Bar, 25 μm.
Figure 4
Representative photomicrographs demonstrating BACE, PS1 and CCA in damaged axons in the subcortical white matter and basal ganglia at 3 days to 6 months following brain injury in the pig. Accumulations in axons were detected by antibodies BACE-2, PS-1, and 249. Bar, 25 μm.
Figure 5
Representative photomicrographs showing Aβ-containing plaque-like profiles in the gray and white matter. Occasionally, perivascualr Aβ depostis were found (top, middle). Aβ accumulation was also found in cortical and cerebellar neurons at 3 days to 6 months following brain trauma. Aβ was identified by antibodies, 6F/3D, Aβ1–40, 13335. Bar, 25 μm.
Figure 6
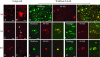
Representative double-immunofluorescence photomicrographs demonstrating co-accumulations of proteins in damaged axons (A–L), neurons (M–N) and macrophages (O–P) at 3 days and 6 months post-injury. Merged green and red fluorescence shown in yellow. In axon bulbs in the white matter, co-accumulation Aβ (antibodies 6F3D and 13335/Green) was found with CCA (249/Red) in (A) and (F), caspase-3 (P20/Red) in (B), BACE (BACE-2/Red) in (G), APP (22C11/Red) in (E), and PS-1 (PS-1/Red) in (H). Co-accumulation of BACE (Green) was found with APP (Red) in (C) and (I), kinesin (L1/Red) in (D) and (K), and CCA (Red) in (J). Co-accumulation of APP (Red) was found with PS-1 (Green) in (L). In neurons, Aβ (Green) co-accumulated with APP (Red) in (M) and CCA (Red) in (N). Macrophages demonstrated co-immunoreactivity of Aβ (13335/Green) with OX42 (CD11b/Red) in (O) and (P). Bar, 25 μm.
Figure 7
Representative photomicrographs showing Congo red staining in damaged axons in subcortical white matter at 3 days, 7 days and 6 months post-injury (A, C, D, E, and F), and in plaque-like profiles in the white matter at 3 and 7 days post-injury (B and D). Congo red stained plaque-like profiles (D, downward arrow) could be found in proximity with axonal bulbs (D, diagonal arrow). Double-staining with Thioflavin S and an anti-Aβ antibody shows co-localization in swollen axons in the subcortical white matter (G–J) at 3 days to 6 months after brain trauma, as well as in a few plaque-like profiles in the gray matter (K) at 6 months following brain injury. Bar, 25 μm.
Figure 8
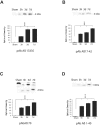
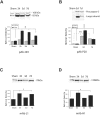
Western blot analysis of Aβ in injured and sham brain white matter. Immunoreactive bands at the molecular weight of Aβ peptides (approximately 4 kd) were found at all post-injury time points by all anti-Aβ antibodies used (primary antibodies listed below graphs). Graphic representation of quantitative analysis of the optical density of the bands used mean values and SE from two separate experiments. P values refer to comparison between sham and groups of injured animals at different time points. ***, P < 0.001.
Figure 9
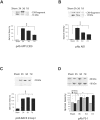
Western blot analysis of APP C99 fragment (14 kd), BACE, and PS-1 in injured and sham brain white matter. Primary antibodies used are listed below graphs. Quantitative analysis of the protein expression is shown graphically, with mean values and SE of optical density of immunostaining from two separate experiments. P values refer to comparison between groups of injured animals and sham. *, P < 0.05; ***, P < 0.001; †, P < 0.05.
Figure 10
Western blot analysis of caspase-mediated APP proteolysis, caspase-3, and kinesins in injured and sham brain white matter. (A) Banding representing CCA, (B) the 20 kd large subunit of caspase-3 and procapase, (C) Kinesin-L, and (D) Kinesin-H. Primary antibodies listed below graphs. Quantitative analysis of the protein expression of CCA, caspase-3, and kinesins is shown graphically with mean values and SE of optical density of immunostaining from two separate experiments. P values refer to comparison between groups of injured animals at different time points and sham. *, P < 0.05; **, P < 0.01; ***, P < 0.001; †††, P < 0.001.
Figure 11
Proposed pathway of Aβ production and dispersal in diffuse axonal injury.
Similar articles
- Caspase-3-mediated cleavage of amyloid precursor protein and formation of amyloid Beta peptide in traumatic axonal injury.
Stone JR, Okonkwo DO, Singleton RH, Mutlu LK, Helm GA, Povlishock JT. Stone JR, et al. J Neurotrauma. 2002 May;19(5):601-14. doi: 10.1089/089771502753754073. J Neurotrauma. 2002. PMID: 12042095 - Long-term accumulation of amyloid-beta in axons following brain trauma without persistent upregulation of amyloid precursor protein genes.
Iwata A, Chen XH, McIntosh TK, Browne KD, Smith DH. Iwata A, et al. J Neuropathol Exp Neurol. 2002 Dec;61(12):1056-68. doi: 10.1093/jnen/61.12.1056. J Neuropathol Exp Neurol. 2002. PMID: 12484568 - Proteolytic processing of the Alzheimer's disease amyloid precursor protein in brain and platelets.
Evin G, Zhu A, Holsinger RM, Masters CL, Li QX. Evin G, et al. J Neurosci Res. 2003 Nov 1;74(3):386-92. doi: 10.1002/jnr.10745. J Neurosci Res. 2003. PMID: 14598315 - BACE1: the beta-secretase enzyme in Alzheimer's disease.
Vassar R. Vassar R. J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review. - The beta-secretase, BACE: a prime drug target for Alzheimer's disease.
Vassar R. Vassar R. J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
Cited by
- Microglial process convergence onto injured axonal swellings, a human postmortem brain tissue study.
Logan-Wesley AL, Gorse KM, Lafrenaye AD. Logan-Wesley AL, et al. Sci Rep. 2024 Sep 12;14(1):21369. doi: 10.1038/s41598-024-71312-7. Sci Rep. 2024. PMID: 39266604 Free PMC article. - Oligodendrocyte birth and death following traumatic brain injury in adult mice.
Dent KA, Christie KJ, Bye N, Basrai HS, Turbic A, Habgood M, Cate HS, Turnley AM. Dent KA, et al. PLoS One. 2015 Mar 23;10(3):e0121541. doi: 10.1371/journal.pone.0121541. eCollection 2015. PLoS One. 2015. PMID: 25798924 Free PMC article. - Damage to myelin and oligodendrocytes: a role in chronic outcomes following traumatic brain injury?
Maxwell WL. Maxwell WL. Brain Sci. 2013 Sep 16;3(3):1374-94. doi: 10.3390/brainsci3031374. Brain Sci. 2013. PMID: 24961533 Free PMC article. - Roadmap for Advancing Pre-Clinical Science in Traumatic Brain Injury.
Smith DH, Kochanek PM, Rosi S, Meyer R, Ferland-Beckham C, Prager EM, Ahlers ST, Crawford F. Smith DH, et al. J Neurotrauma. 2021 Dec;38(23):3204-3221. doi: 10.1089/neu.2021.0094. Epub 2021 Aug 13. J Neurotrauma. 2021. PMID: 34210174 Free PMC article. Review. - Traumatic Brain Injury and Risk of Neurodegenerative Disorder.
Brett BL, Gardner RC, Godbout J, Dams-O'Connor K, Keene CD. Brett BL, et al. Biol Psychiatry. 2022 Mar 1;91(5):498-507. doi: 10.1016/j.biopsych.2021.05.025. Epub 2021 Jun 2. Biol Psychiatry. 2022. PMID: 34364650 Free PMC article. Review.
References
- Rasmusson DX, Brandt J, Martin DB. Head injury as a risk factor in Alzheimer’s disease. Brain Inj. 1995;9:213–219. - PubMed
- Nemetz PN, Leibso C, Naessens JM. Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. Am J Epidemiol. 1999;149:32–40. - PubMed
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips BA, Gau BA, Welsh-Bohmer KA, Burke JR, Guralink JM, Breitner JC. Documented head injury in early adulthood and risk of Alzheimer’s disease dementias. Neurology. 2000;8:1158–1166. - PubMed
- Lye TC, Shores EA. Traumatic brain injury as a risk factor for Alzheimer’s disease: a review. Neuropsychol Rev. 2000;10:115–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS08803/NS/NINDS NIH HHS/United States
- NS38104/NS/NINDS NIH HHS/United States
- P01 AG011542/AG/NIA NIH HHS/United States
- R01 NS038104/NS/NINDS NIH HHS/United States
- AG11542/AG/NIA NIH HHS/United States
- P50 NS008803/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials