Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis - PubMed (original) (raw)
Comparative Study
Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis
Kathleen R Lamborn et al. Neuro Oncol. 2004 Jul.
Abstract
Survival for patients with glioblastoma multiforme is short, and current treatments provide limited benefit. Therefore, there is interest in conducting phase 2 trials of experimental treatments in newly diagnosed patients. However, this requires historical data with which to compare the experimental therapies. Knowledge of prognostic markers would also allow stratification into risk groups for phase 3 randomized trials. In this retrospective study of 832 glioblastoma multiforme patients enrolled into prospective clinical trials at the time of initial diagnosis, we evaluated several potential prognostic markers for survival to establish risk groups. Analyses were done using both Cox proportional hazards modeling and recursive partitioning analyses. Initially, patients from 8 clinical trials, 6 of which included adjuvant chemotherapy, were included. Subsequent analyses excluded trials with interstitial brachytherapy, and finally included only nonbrachytherapy trials with planned adjuvant chemotherapy. The initial analysis defined 4 risk groups. The 2 lower risk groups included patients under the age of 40, the lowest risk group being young patients with tumor in the frontal lobe only. An intermediate-risk group included patients with Karnofsky performance status (KPS) >70, subtotal or total resection, and age between 40 and 65. The highest risk group included all patients over 65 and patients between 40 and 65 with either KPS<80 or biopsy only. Subgroup analyses indicated that inclusion of adjuvant chemotherapy provides an increase in survival, although that improvement tends to be minimal for patients over age 65, for patients over age 40 with KPS less than 80, and for those treated with brachytherapy.
Figures
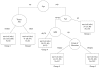
Fig. 1
Recursive partitioning analysis for all patients (N = 832). For terminal nodes (□), median survival and the 95% confidence interval for the median are given. N = number of patients. Estimates with an asterisk (*) exclude 40 patients missing KPS and 2 patients missing extent of resection.
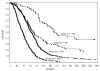
Fig. 2
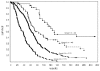
Survival for all patients, by risk group. Fourteen patients in group 1, 12 patients in group 2, and 9 patients in group 3 lived beyond 5 years (260 weeks). Individual survival times (including those for patients who are censored) are indicated by a solid circle (•). Fifty-three patients were censored (13 in group 1, 14 in group 2, 21 in group 3, and 5 in group 4). Additional detail is given in Table 4.
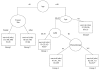
Fig. 3
Recursive partitioning analysis for all patients on non-brachytherapy protocols (n = 660). For terminal nodes (□), median survival and the 95% confidence interval for the median are given. N = number of patients. *Twenty-eight patients without KPS were excluded from the estimates.
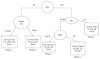
Fig. 4
Recursive partitioning analysis for all patients on non-brachytherapy protocols that included adjuvant chemotherapy (n = 437). For terminal nodes (□), median survival and the 95% confidence interval for the median are given. N = number of patients. *Twenty-eight patients with missing KPS were excluded from the estimates.
Fig. 5
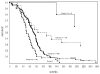
Survival for patients on nonbrachytherapy protocols that included adjuvant chemotherapy. Nine patients in group 1, 6 patients in group 2, and 7 patients in group 3 survived beyond 5 years (260 weeks). Individual survival times (including those for patients that are censored) are indicated by a solid circle (•). Forty-four patients were censored (12 in group 1, 8 in group 2, 21 in group 3, and 3 in group 4). Additional detail is given in Table 6.
Fig. 6
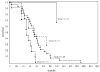
Survival for all patients on brachytherapy protocols (n = 160). Risk groups are as defined in Table 4 for all patients. Three patients in group 1 and 5 patients in group 2 lived beyond 5 years (260 weeks). Individual survival times (including those for patients that are censored) are indicated by a solid circle (•). Eight patients were censored (1 in group 1, 5 in group 2, 2 in group 3, and 0 in group 4).
Fig. 7
Survival for patients on brachytherapy protocols that included adjuvant chemotherapy (n = 49). Risk groups are as defined for all patients in Table 4. Two patients in group 1 and 2 patients in group 2 lived beyond 5 years (260 weeks). Individual survival times (including those for patients that are censored) are indicated by a solid circle (•). Two patients were censored (both in group 2).
Similar articles
- Treatment outcomes for patients with glioblastoma multiforme and a low Karnofsky Performance Scale score on presentation to a tertiary care institution. Clinical article.
Marina O, Suh JH, Reddy CA, Barnett GH, Vogelbaum MA, Peereboom DM, Stevens GH, Elinzano H, Chao ST. Marina O, et al. J Neurosurg. 2011 Aug;115(2):220-9. doi: 10.3171/2011.3.JNS10495. Epub 2011 May 6. J Neurosurg. 2011. PMID: 21548745 - Role of adjuvant or salvage radiosurgery in the management of unresected residual or progressive glioblastoma multiforme in the pre-bevacizumab era.
Niranjan A, Kano H, Iyer A, Kondziolka D, Flickinger JC, Lunsford LD. Niranjan A, et al. J Neurosurg. 2015 Apr;122(4):757-65. doi: 10.3171/2014.11.JNS13295. Epub 2015 Jan 16. J Neurosurg. 2015. PMID: 25594327 - Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials.
Carson KA, Grossman SA, Fisher JD, Shaw EG. Carson KA, et al. J Clin Oncol. 2007 Jun 20;25(18):2601-6. doi: 10.1200/JCO.2006.08.1661. J Clin Oncol. 2007. PMID: 17577040 Free PMC article. - Effectiveness of maximal safe resection for glioblastoma including elderly and low Karnofsky performance status patients: retrospective review at a single institute.
Uzuka T, Aoki H, Natsumeda M, Takahashi H, Fujii Y. Uzuka T, et al. Neurol Med Chir (Tokyo). 2012;52(8):570-6. doi: 10.2176/nmc.52.570. Neurol Med Chir (Tokyo). 2012. PMID: 22976140 - Clinical oncology research; Review on contemporary methodology standards.
Nasr MM, Nasr MM, Shehata LH. Nasr MM, et al. Curr Probl Cancer. 2021 Oct;45(5):100725. doi: 10.1016/j.currproblcancer.2021.100725. Epub 2021 Mar 6. Curr Probl Cancer. 2021. PMID: 33715867 Review.
Cited by
- Radiomics-Based Machine Learning with Natural Gradient Boosting for Continuous Survival Prediction in Glioblastoma.
Karabacak M, Patil S, Gersey ZC, Komotar RJ, Margetis K. Karabacak M, et al. Cancers (Basel). 2024 Oct 26;16(21):3614. doi: 10.3390/cancers16213614. Cancers (Basel). 2024. PMID: 39518054 Free PMC article. - Prognostic values of combined ratios of white blood cells in glioma: a systematic review and meta-analysis.
Zhou J, Tan B, Gao F. Zhou J, et al. Neurosurg Rev. 2024 Oct 31;47(1):831. doi: 10.1007/s10143-024-03064-x. Neurosurg Rev. 2024. PMID: 39477886 - Molecular Profile as an Outcome Predictor in Glioblastoma along with MRI Features and Surgical Resection: A Scoping Review.
Papacocea SI, Vrinceanu D, Dumitru M, Manole F, Serboiu C, Papacocea MT. Papacocea SI, et al. Int J Mol Sci. 2024 Sep 8;25(17):9714. doi: 10.3390/ijms25179714. Int J Mol Sci. 2024. PMID: 39273661 Free PMC article. Review. - Resection versus biopsy in patients with glioblastoma (RESBIOP study): study protocol for an international multicentre prospective cohort study (ENCRAM 2202).
Gerritsen JKW, Young JS, Krieg SM, Jungk C, Ille S, Schucht P, Nahed BV, Broekman MLD, Berger M, De Vleeschouwer S, Vincent AJPE. Gerritsen JKW, et al. BMJ Open. 2024 Sep 10;14(9):e081689. doi: 10.1136/bmjopen-2023-081689. BMJ Open. 2024. PMID: 39260848 Free PMC article. - Supramaximal resection: retrospective study on IDH-wildtype Glioblastomas based on the new RANO-Resect classification.
Tropeano MP, Raspagliesi L, Bono BC, Baram A, Rossini Z, Franzini A, Navarria P, Clerici E, Bellu L, Simonelli M, Scorsetti M, Riva M, Politi LS, Pessina F. Tropeano MP, et al. Acta Neurochir (Wien). 2024 Apr 27;166(1):196. doi: 10.1007/s00701-024-06090-2. Acta Neurochir (Wien). 2024. PMID: 38676720
References
- Breiman, L., Friedman, J.H., Olshen, R.A., and Stone, C.J. (1984) Classification and Regression Trees Belmont, Calif.: Wadsworth International Group.
- Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE, Nelson DF. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. - PubMed
- GMT. Glioma Meta-analysis Trialists (GMT) Group. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. - PubMed
- Keles S, Segal MR. Residual-based tree-structured survival analysis. Stat Med. 2002;21:313–326. - PubMed
- Laperriere NJ, Leung PMK, McKenzie S, Milosevic M, Wong S, Glen J, Pintilie M, Bernstein M. Randomized study of brachytherapy in the initial management of patients with malignant astrocytoma. Int J Radiat Oncol Biol Phys. 1998;41:1005–1011. - PubMed
Publication types
MeSH terms
Grants and funding
- CA82103/CA/NCI NIH HHS/United States
- NS42927/NS/NINDS NIH HHS/United States
- P30 CA082103/CA/NCI NIH HHS/United States
- P50 CA097257/CA/NCI NIH HHS/United States
- P01 NS042927/NS/NINDS NIH HHS/United States
- CA097257/CA/NCI NIH HHS/United States
- CA13525/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical