Beyond help: direct effector functions of human immunodeficiency virus type 1-specific CD4(+) T cells - PubMed (original) (raw)
Beyond help: direct effector functions of human immunodeficiency virus type 1-specific CD4(+) T cells
Philip J Norris et al. J Virol. 2004 Aug.
Abstract
The immune correlates of protection in human immunodeficiency virus type 1 (HIV-1) infection remain poorly defined, particularly the contribution of CD4(+) T cells. Here we explore the effector functions of HIV-1-specific CD4(+) T cells. We demonstrate HIV-1 p24-specific CD4(+)-T-cell cytolytic activity in peripheral blood mononuclear cells directly ex vivo and after enrichment by antigen-specific stimulation. We further show that in a rare long-term nonprogressor, both an HIV-1-specific CD4(+)-T-cell clone and CD4(+) T cells directly ex vivo exert potent suppression of HIV-1 replication. Suppression of viral replication was dependent on cell-cell contact between the effector CD4(+) T cells and the target cells. While the antiviral effector activity of CD8(+) T cells has been well documented, these results strongly suggest that HIV-1-specific CD4(+) T cells are capable of directly contributing to antiviral immunity.
Figures
FIG. 1.
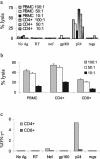
Ex vivo CD4+-T-cell lysis. (a) The ability of freshly isolated PBMC or CD8+-T-cell-depleted T cells to kill B-cell targets pulsed with HIV-1 or control proteins (reverse transcriptase, Nef, gp160, and mgs) or a peptide pool (p24) was tested in an overnight killing assay. The only HIV-1 antigen to elicit significant lysis was the peptide pool spanning p24, and depletion of CD8+ cells increased lysis at a given E:T ratio. The CD4+-T-cell-enriched population was 89.1% CD3+ CD4+ cells and 6.5% CD3+ CD8+ cells (data not shown). E:T ratios are noted in the legend of each figure. Spontaneous lysis was 33% for the mgs-pulsed B-LCL and 41% for the p24-pulsed B-LCL. Of note, spontaneous lysis of p24-pulsed B-LCL was 17% in the subsequent experiment analyzed in panel b, and there was no change in the outcome of the experiment. We confirmed the ex vivo cytolytic activity in subject 161J on six separate occasions. (b) Enrichment for CD4+ or CD8+ T cells and lysis of targets pulsed with whole p24 protein. CD4+ refers to PBMC enriched for CD4+ T cells during isolation, and CD8+ refers to PBMC enriched for CD8+ T cells. Enrichment for CD4+ T cells led to an increase in lysis, and enrichment for CD8+ T cells led to diminution of lysis. The CD8+-T-cell-enriched population was 88.8% CD3+ CD8+ and 3.0% CD3+ CD4+; the purity of the CD4+-T-cell-enriched population was not tested. (c) Frequency of HIV-1-specific cells ex vivo. Fresh PBMC were stimulated with the indicated whole proteins at a concentration of 5 μg/ml except for p24, where a pool of 18-amino-acid peptides spanning p24 were used as the stimulus. Cells were stained for production of IFN-γ and quantitated by flow cytometry. At least 50,000 live events/condition were recorded. Since whole protein was used as a stimulus for each of the conditions except p24, CD8+-T-cell responses would be expected to be low.
FIG. 2.
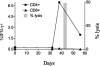
Lysis by a T-cell line from patient AC-25. p24-specific lysis was assayed directly ex vivo and at 42 days with a T-cell line. CD4+ and CD8+ T cells producing IFN-γ after stimulation with p24 are shown ex vivo and at three subsequent time points. At least 15,000 and generally more than 40,000 live events were collected/condition. Lysis was demonstrated after the level of p24-specific CD4+ T cells rose above 6%.
FIG. 3.
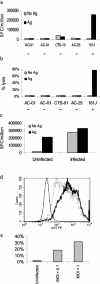
Recognition of HIV-1-infected MT-2 cells by an HLA-matched Th-cell clone. (a) MT-2 cells were incubated with a mixture of cognate epitopes for all five of the Th-cell clones. Only the HLA-matched, DR4-restricted clone could recognize antigen presented by the MT-2 cells. The − and + signs below each clone indicate whether or not the clone was HLA matched to the target cell. Positive controls included stimulation with autologous B-LCL pulsed with cognate antigen, confirming each clone's activity (data not shown). (b) 161J clone can lyse MT-2 cells in an antigen-specific fashion. Only the HLA-matched Th-cell clone could lyse MT-2 cells in a 4-h 51Cr release assay. Clonal activity was confirmed in parallel with autologous B-LCL targets pulsed with cognate antigen for each clone (data not shown). (c) Infected MT-2 cells process antigen for recognition by the Th-cell clone 161J. Infected and uninfected cells were used as antigen-presenting cells in an overnight ELISPOT assay for IFN-γ secretion. Cells were infected at an MOI of 1. The uninfected cells induced IFN-γ secretion only if exogenous cognate antigen was added, while the infected cells needed no exogenous antigen to induce IFN-γ secretion. (d) Granular staining of infected MT-2 cells for intracellular p24. The thin histogram represents uninfected cells, the dashed histogram represents cells infected at an MOI of 0.1, and the bold histogram represents cells infected at an MOI of 1. The mean fluorescence intensities for each of the conditions, respectively, were 19.3, 825, and 1032. (e) Lysis of infected MT-2 cells. The 161J clone was able to lyse infected MT-2 cells in a 4-h 51Cr release assay, with greater lysis in targets expressing higher levels of p24 as determined in Fig. 3d.
FIG. 4.
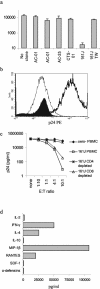
Suppression of HIV-1 replication and secretion of antiviral factors. (a) MT-2 cells were infected with HIV-1 IIIB, and the supernatant was assayed for p24 production by ELISA at day 7. Each of the clones was added at a 1:1 E:T ratio. Only the HLA-matched 161J clone was able to suppress virus replication. Suppression depended on cell-cell contact, since separation of the clone from the targets with a semipermeable membrane (transwell [TW]) abrogated suppression. Results are averages of at least two independent experiments, and error bars represent the standard error. (b) Intracellular staining of MT-2 cells for p24 at day 11 after infection. MT-2 cells were gated based on their large size and granularity in the forward and side scatter gates. The thin histogram represents uninfected cells, the thick histogram represents infected cells, and the filled histogram represents infected cells incubated with the 161J Th-cell clone at an E:T ratio of 1:1. The mean fluorescence intensities for each of the conditions, respectively, were 27.0, 1670, and 32.3. More than 50,000 events/condition were recorded, except for the infected cells alone, where 22,000 events were acquired due to lower viable cell numbers by day 11. (c) Suppression of HIV-1 replication by 161J PBMC directly ex vivo. Infected MT-2 cells were incubated with various E:T ratios of 161J PBMC or PBMC depleted of CD4+ or CD8+ T cells, and p24 was quantitated by ELISA at day 7. Virus was suppressed by PBMC and the CD4+-T-cell subsets but not PBMC depleted of CD4+ T cells. No virus suppression was demonstrated after coincubation with PBMC from a control HIV-1-seronegative individual. (d) Secretion of cytokines and antiviral factors by 161J Th-cell clone. Clone cells were stimulated with a cognate antigen at 5 μg/ml presented by an autologous B-cell line, and supernatants were harvested at 24, 48, and 72 h. The highest value of the three for each cytokine is listed. IL-4, IFN-γ, and the CCR5 chemokines were secreted at the highest levels.
Similar articles
- Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+, cytotoxic phenotype in an HIV-1 long-term nonprogressor and in CMV infection.
Zaunders JJ, Dyer WB, Wang B, Munier ML, Miranda-Saksena M, Newton R, Moore J, Mackay CR, Cooper DA, Saksena NK, Kelleher AD. Zaunders JJ, et al. Blood. 2004 Mar 15;103(6):2238-47. doi: 10.1182/blood-2003-08-2765. Epub 2003 Nov 26. Blood. 2004. PMID: 14645006 - Strong ability of Nef-specific CD4+ cytotoxic T cells to suppress human immunodeficiency virus type 1 (HIV-1) replication in HIV-1-infected CD4+ T cells and macrophages.
Zheng N, Fujiwara M, Ueno T, Oka S, Takiguchi M. Zheng N, et al. J Virol. 2009 Aug;83(15):7668-77. doi: 10.1128/JVI.00513-09. Epub 2009 May 20. J Virol. 2009. PMID: 19457989 Free PMC article. - Perspectives on inducing efficient immune control of HIV-1 replication--a new goal for HIV therapeutics?
Bucy RP, Kilby JM. Bucy RP, et al. AIDS. 2001 Feb;15 Suppl 2:S36-42. doi: 10.1097/00002030-200102002-00007. AIDS. 2001. PMID: 11424975 Review.
Cited by
- Cell-mediated protection in influenza infection.
Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Thomas PG, et al. Emerg Infect Dis. 2006 Jan;12(1):48-54. doi: 10.3201/eid1201.051237. Emerg Infect Dis. 2006. PMID: 16494717 Free PMC article. Review. - Possible clearance of transfusion-acquired nef/LTR-deleted attenuated HIV-1 infection by an elite controller with CCR5 Δ32 heterozygous and HLA-B57 genotype.
Zaunders J, Dyer WB, Churchill M, Munier CML, Cunningham PH, Suzuki K, McBride K, Hey-Nguyen W, Koelsch K, Wang B, Hiener B, Palmer S, Gorry PR, Bailey M, Xu Y, Danta M, Seddiki N, Cooper DA, Saksena NK, Sullivan JS, Riminton S, Learmont J, Kelleher AD. Zaunders J, et al. J Virus Erad. 2019 Apr 1;5(2):73-83. doi: 10.1016/S2055-6640(20)30696-11. J Virus Erad. 2019. PMID: 31191910 Free PMC article. - Characterization of a library of 20 HBV-specific MHC class II-restricted T cell receptors.
Schreiber S, Honz M, Mamozai W, Kurktschiev P, Schiemann M, Witter K, Moore E, Zielinski C, Sette A, Protzer U, Wisskirchen K. Schreiber S, et al. Mol Ther Methods Clin Dev. 2021 Oct 29;23:476-489. doi: 10.1016/j.omtm.2021.10.012. eCollection 2021 Dec 10. Mol Ther Methods Clin Dev. 2021. PMID: 34853796 Free PMC article. - Gag- and Nef-specific CD4+ T cells recognize and inhibit SIV replication in infected macrophages early after infection.
Sacha JB, Giraldo-Vela JP, Buechler MB, Martins MA, Maness NJ, Chung C, Wallace LT, León EJ, Friedrich TC, Wilson NA, Hiraoka A, Watkins DI. Sacha JB, et al. Proc Natl Acad Sci U S A. 2009 Jun 16;106(24):9791-6. doi: 10.1073/pnas.0813106106. Epub 2009 May 28. Proc Natl Acad Sci U S A. 2009. PMID: 19478057 Free PMC article. - CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system.
Sitati EM, Diamond MS. Sitati EM, et al. J Virol. 2006 Dec;80(24):12060-9. doi: 10.1128/JVI.01650-06. Epub 2006 Oct 11. J Virol. 2006. PMID: 17035323 Free PMC article.
References
- Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. - PMC - PubMed
- Appay, V., J. J. Zaunders, L. Papagno, J. Sutton, A. Jaramillo, A. Waters, P. Easterbrook, P. Grey, D. Smith, A. J. McMichael, D. A. Cooper, S. L. Rowland-Jones, and A. D. Kelleher. 2002. Characterization of CD4(+) CTLs ex vivo. J. Immunol. 168:5954-5958. - PubMed
- Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV-1)-specific CD4(+) and CD8(+) t-cell responses: relationship to viral load in untreated HIV-1 infection. J. Virol. 75:11983-11991. - PMC - PubMed
- Bitmansour, A. D., S. L. Waldrop, C. J. Pitcher, E. Khatamzas, F. Kern, V. C. Maino, and L. J. Picker. 2001. Clonotypic structure of the human CD4+ memory T cell response to cytomegalovirus. J. Immunol. 167:1151-1163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI-040873/AI/NIAID NIH HHS/United States
- R01 AI040873/AI/NIAID NIH HHS/United States
- K08 AI001698/AI/NIAID NIH HHS/United States
- R21 AI040873/AI/NIAID NIH HHS/United States
- AI-01698-01/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials