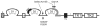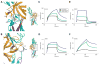Biochemical analysis of pathogenic ligand-dependent FGFR2 mutations suggests distinct pathophysiological mechanisms for craniofacial and limb abnormalities - PubMed (original) (raw)
. 2004 Oct 1;13(19):2313-24.
doi: 10.1093/hmg/ddh235. Epub 2004 Jul 28.
Affiliations
- PMID: 15282208
- PMCID: PMC4140565
- DOI: 10.1093/hmg/ddh235
Biochemical analysis of pathogenic ligand-dependent FGFR2 mutations suggests distinct pathophysiological mechanisms for craniofacial and limb abnormalities
Omar A Ibrahimi et al. Hum Mol Genet. 2004.
Abstract
Gain-of-function missense mutations in FGF receptor 2 (FGFR2) are responsible for a variety of craniosynostosis syndromes including Apert syndrome (AS), Pfeiffer syndrome (PS) and Crouzon syndrome (CS). Unlike the majority of FGFR2 mutations, S252W and P253R AS mutations and a D321A PS mutation retain ligand-dependency and are also associated with severe limb pathology. In addition, a recently identified ligand-dependent S252L/A315S double mutation in FGFR2 was shown to cause syndactyly in the absence of craniosynostosis. Here, we analyze the effect of the canonical AS mutations, the D321A PS mutation and the S252L/A315S double mutation on FGFR2 ligand binding affinity and specificity using surface plasmon resonance. Both AS mutations and the D321A PS mutation, but not the S252L/A315S double mutation, increase the binding affinity of FGFR2c to multiple FGFs expressed in the cranial suture. Additionally, all four pathogenic mutations also violate FGFR2c ligand binding specificity and enable this receptor to bind FGF10. Based on our data, we propose that an increase in mutant FGFR2c binding to multiple FGFs results in craniosynostosis, whereas binding of mutant FGFR2c to FGF10 results in severe limb pathology. Structural and biophysical analysis shows that AS mutations in FGFR2b also enhance and violate FGFR2b ligand binding affinity and specificity, respectively. We suggest that elevated AS mutant FGFR2b signaling may account for the dermatological manifestations of AS.
Figures
Figure 1
Mapping of pathogenic FGFR2 mutations. D1, D2, D3 represent immunoglobulin (Ig)-like domain 1, 2 and 3; S represents the signal peptide; AB represents the acid box; TM represents the transmembrane helix; TK1 and TK2 represent the split-kinase domain, which is interrupted by the kinase insert; J represents the juxtamembrane region. The heparin-binding site (HBS) in D2 is marked by a thickened black line. The alternatively spliced region (encoded by either exon ‘b’ or ‘c’ in a tissue specific manner) in D3 is represented by a thickened gray line. The location of AS S252W and P253R mutations, the D321A PS mutation and the S252L/A315S double mutation are indicated by arrows. The D321A PS mutation and the S252L/A315S double mutation manifest only in the ‘c’ isoform of FGFR2.
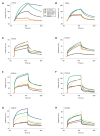
Figure 2
Surface plasmon resonance analysis of wild-type and mutant FGFR2c-FGF interactions. Sensorgrams of representative analyte injections of wild-type, S252W, P253R, D321A and S252L/A315S FGFR2c binding to (A) FGF2 (at 12.5 nM), (B) FGF4 (at 6.25 nM), (C) FGF5 (at 200 nM), (D) FGF10 (at 400 nM), (E) FGF16 (at 200 nM), (F) FGF18 (at 400 nM), (G) FGF19 (at 400 nM), and (H) FGF23 (at 400 nM). Analyte injections are colored as follows: wild-type FGFR2c in black, S252W FGFR2c in blue, P253R FGFR2c in green, D321A FGFR2c in yellow and S252L/A315S in red. The biosensor chip response is indicated on the _y_-axis (ΔRU) as a function of time (_x_-axis) at 25°C. Kinetic data are summarized in Table 1.
Figure 3
Structural and biophysical analysis of wild-type and AS mutant FGFR2b-FGF interactions. (A) Gain-of-function contact in the S252W FGFR2b-FGF10 complex. D2 and D3 of FGFR2b are shown in green and cyan, respectively. The alternatively spliced region of D3 is colored purple. The D2–D3 linker is colored gray. FGF10 is shown in orange. (Right) View of whole structure in the exact orientation as the detailed view is shown, with the region of interest boxed. (B) Gain-of-function hydrogen bonds in the P253R FGFR2b-FGF1 complex. Coloring is as in (A). Dotted lines represent hydrogen bonds and the hydrogen-bonding distances are indicated. (Right) View of whole structure in the exact orientation as the detailed view is shown, with the region of interest boxed. (C, D, E and F) Sensorgrams of representative analyte injections of wild-type and AS mutant FGFR2b binding to (C) FGF10 (at 100 nM), (D) FGF1 (at 400 nM), (E) FGF2 (at 400 nM), and (F) FGF8 (at 800 nM). Analyte injections are colored as follows: wild-type FGFR2b in black, S252W FGFR2b in blue, and P253R FGFR2b in green. The biosensor chip response is indicated on the _y_-axis (ΔRU) as a function of time (_x_-axis) at 25°C. Kinetic data are summarized in Table 2.
Similar articles
- Proline to arginine mutations in FGF receptors 1 and 3 result in Pfeiffer and Muenke craniosynostosis syndromes through enhancement of FGF binding affinity.
Ibrahimi OA, Zhang F, Eliseenkova AV, Linhardt RJ, Mohammadi M. Ibrahimi OA, et al. Hum Mol Genet. 2004 Jan 1;13(1):69-78. doi: 10.1093/hmg/ddh011. Epub 2003 Nov 12. Hum Mol Genet. 2004. PMID: 14613973 - Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome.
Yu K, Herr AB, Waksman G, Ornitz DM. Yu K, et al. Proc Natl Acad Sci U S A. 2000 Dec 19;97(26):14536-41. doi: 10.1073/pnas.97.26.14536. Proc Natl Acad Sci U S A. 2000. PMID: 11121055 Free PMC article. - FGFs, their receptors, and human limb malformations: clinical and molecular correlations.
Wilkie AO, Patey SJ, Kan SH, van den Ouweland AM, Hamel BC. Wilkie AO, et al. Am J Med Genet. 2002 Oct 15;112(3):266-78. doi: 10.1002/ajmg.10775. Am J Med Genet. 2002. PMID: 12357470 Review. - Craniosynostosis and related limb anomalies.
Wilkie AO, Oldridge M, Tang Z, Maxson RE Jr. Wilkie AO, et al. Novartis Found Symp. 2001;232:122-33; discussion 133-43. doi: 10.1002/0470846658.ch9. Novartis Found Symp. 2001. PMID: 11277076 Review.
Cited by
- Quantitative and qualitative differences in the activation of a fibroblast growth factor receptor by different FGF ligands.
Krzyscik MA, Karl K, Dudeja P, Krejci P, Hristova K. Krzyscik MA, et al. Cytokine Growth Factor Rev. 2024 Aug;78:77-84. doi: 10.1016/j.cytogfr.2024.07.002. Epub 2024 Jul 9. Cytokine Growth Factor Rev. 2024. PMID: 39043538 Review. - Signaling Pathway and Small-Molecule Drug Discovery of FGFR: A Comprehensive Review.
Zheng J, Zhang W, Li L, He Y, Wei Y, Dang Y, Nie S, Guo Z. Zheng J, et al. Front Chem. 2022 Apr 14;10:860985. doi: 10.3389/fchem.2022.860985. eCollection 2022. Front Chem. 2022. PMID: 35494629 Free PMC article. Review. - Excessive osteoclast activation by osteoblast paracrine factor RANKL is a major cause of the abnormal long bone phenotype in Apert syndrome model mice.
Shin HR, Kim BS, Kim HJ, Yoon H, Kim WJ, Choi JY, Ryoo HM. Shin HR, et al. J Cell Physiol. 2022 Apr;237(4):2155-2168. doi: 10.1002/jcp.30682. Epub 2022 Jan 20. J Cell Physiol. 2022. PMID: 35048384 Free PMC article. - Acne Syndromes and Mosaicism.
Baroud S, Wu J, Zouboulis CC. Baroud S, et al. Biomedicines. 2021 Nov 21;9(11):1735. doi: 10.3390/biomedicines9111735. Biomedicines. 2021. PMID: 34829964 Free PMC article. Review. - Activation of FGFR2 Signaling Suppresses BRCA1 and Drives Triple-Negative Mammary Tumorigenesis That is Sensitive to Immunotherapy.
Lei JH, Lee MH, Miao K, Huang Z, Yao Z, Zhang A, Xu J, Zhao M, Huang Z, Zhang X, Chen S, Jiaying NG, Feng Y, Xing F, Chen P, Sun H, Chen Q, Xiang T, Chen L, Xu X, Deng CX. Lei JH, et al. Adv Sci (Weinh). 2021 Nov;8(21):e2100974. doi: 10.1002/advs.202100974. Epub 2021 Sep 13. Adv Sci (Weinh). 2021. PMID: 34514747 Free PMC article.
References
- Cohen MM., Jr . Craniosynostosis, Diagnosis, Evaluation, and Management. Oxford Univeristy Press; New York: 2000.
- Muenke M, Wilkie AO. Craniosynostosis syndromes. In: Scriver CR, Beaudet AL, Valle D, Sly WS, Childs Kinzler K, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. IV. McGraw-Hill; New York, NY: 2001. pp. 6117–6146.
- Jabs EW. Genetic etiologies of craniosynostosis. In: Mooney MK, Siegel MI, editors. Understanding Craniofacial Anomalies: The Etiopathogenesis of Craniosynostoses and Facial Clefting. Wiley-Liss; New York: 2002. pp. 125–146.
- Cohen MM, Jr, Kreiborg S. The central nervous system in the Apert syndrome. Am J Med Genet. 1990;35:36–45. - PubMed
- Cohen MM, Jr, Kreiborg S. Cutaneous manifestations of Apert syndrome. Am J Med Genet. 1995;58:94–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL062244-06/HL/NHLBI NIH HHS/United States
- R01 HL062244-05A1/HL/NHLBI NIH HHS/United States
- HL52622/HL/NHLBI NIH HHS/United States
- R01 HL052622/HL/NHLBI NIH HHS/United States
- R01 HL062244/HL/NHLBI NIH HHS/United States
- R01 HL052622-07/HL/NHLBI NIH HHS/United States
- R01 GM038060/GM/NIGMS NIH HHS/United States
- R01 GM038060-16A2/GM/NIGMS NIH HHS/United States
- DE13686/DE/NIDCR NIH HHS/United States
- R01 DE013686/DE/NIDCR NIH HHS/United States
- R01 HL052622-06A1/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous