Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects - PubMed (original) (raw)
. 2004 Aug;114(3):379-88.
doi: 10.1172/JCI21102.
Magali Terme, Julien Taïeb, Cédric Ménard, Caroline Flament, Caroline Robert, Koji Maruyama, Hiro Wakasugi, Eric Angevin, Kris Thielemans, Axel Le Cesne, Véronique Chung-Scott, Vladimir Lazar, Isabelle Tchou, Florent Crépineau, François Lemoine, Jacky Bernard, Jonhantan A Fletcher, Ali Turhan, Jean-Yves Blay, Alain Spatz, Jean-François Emile, Michael C Heinrich, Salah Mécheri, Thomas Tursz, Laurence Zitvogel
Affiliations
- PMID: 15286804
- PMCID: PMC489961
- DOI: 10.1172/JCI21102
Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects
Christophe Borg et al. J Clin Invest. 2004 Aug.
Abstract
Mutant isoforms of the KIT or PDGF receptors expressed by gastrointestinal stromal tumors (GISTs) are considered the therapeutic targets for STI571 (imatinib mesylate; Gleevec), a specific inhibitor of these tyrosine kinase receptors. Case reports of clinical efficacy of Gleevec in GISTs lacking the typical receptor mutations prompted a search for an alternate mode of action. Here we show that Gleevec can act on host DCs to promote NK cell activation. DC-mediated NK cell activation was triggered in vitro and in vivo by treatment of DCs with Gleevec as well as by a loss-of-function mutation of KIT. Therefore, tumors that are refractory to the antiproliferative effects of Gleevec in vitro responded to Gleevec in vivo in an NK cell-dependent manner. Longitudinal studies of Gleevec-treated GIST patients revealed a therapy-induced increase in IFN-gamma production by NK cells, correlating with an enhanced antitumor response. These data point to a novel mode of antitumor action for Gleevec.
Figures
Figure 1
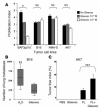
Gleevec prevented tumor progression in vivo in tumor models resistant to the Gleevec antiproliferative effects in vitro. (A) Mouse tumor models resistant to Gleevec in vitro. AK7, B16F10 (B16), RMA-S, MCA 102, or BAF3p210 cells (bearing the BCR/ABL translocation) were incubated for 24 hours with the indicated doses of Gleevec, and the absolute number of surviving cells was determined by trypan blue exclusion assay. Proliferation indexes are shown. The Wilcoxon two-sample rank sum test was used to compare the proliferation indexes (*P < 0.05). (B) Gleevec prevents establishment of B16F10 lung metastases. We injected 5 × 105 B16F10 tumor cells in the tail vein at day 0. Oral feeding with Gleevec (150 mg/kg bid) or H2O (200 μl) was administered on days 5–11 and mice were sacrificed for the enumeration of lung metastases on day 11. The data from 3 independent experiments, each including 5–7 mice per group, were pooled and are depicted. The Wilcoxon two-sample rank sum test was used to compare the number of lung metastases (**P < 0.05, Gleevec versus H2O). (C) FL and Gleevec synergize to eradicate AK7. We inoculated 3 × 106 AK7 tumor cells in the abdominal flank of C57BL/6 mice on day 0. FL was started at day 11 when AK7 tumors reached a diameter of 20 ± 20 mm2. FL was continued for 10 days and combined with Gleevec the day before FL arrest and for 8 consecutive days (same doses as in B). Each experiment included 5–7 mice/group and was repeated twice with similar results. The Kruskal Wallis multiple comparison test was used for statistical analyses and significant effects are signified by triple asterisks.
Figure 2
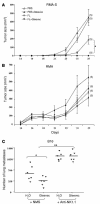
Gleevec promoted NK cell–dependent antitumor effects. (A and B) FL and Gleevec synergize to prevent RMA-S tumor establishment. In one abdominal flank of C57BL/6 mice, TAP-deficient RMA-S cells were injected (A), whereas TAP-sufficient RMA cells were injected into the other flank of the same mice (B). The same number of cells of each type were injected (106 cells). From 6 days before injection (day –6) to day 3, FL (10 μg/day) or PBS (200 μl) was injected intraperitoneally. From day 1 to day 4, Gleevec (150 mg/kg bid) or H2O (200 μl) was administered orally. The number of tumor-free mice at the end of the experiment is indicated in parentheses. Each experiment included 5 mice per group and was repeated twice with similar results. (C) The antitumor effects of Gleevec against B16F10 lung metastases are mediated by NK cells. Neutralizing anti-NK1.1 mAb (300 μg of PK136 mAb/mouse) or normal mouse serum (NMS) were administered intraperitoneally at days –4, -–2, 0, and 4 in C57BL/6 mice. At day 0, 5 × 105 B16F10 tumor cells were inoculated into the tail vein. Oral feeding with Gleevec (150 mg/kg bid) or H2O (200 μl) was administered on days 5–11, and mice were sacrificed for the enumeration of lung metastases at day 11. The results of one representative experiment (of 2) including 5–7 mice per group are shown. The Kruskal Wallis multiple comparison test was used to compare the number of lung metastases (*P < 0.05 between Gleevec and H2O in NMS groups; **P < 0.01 between NMS and anti-NK1.1 mAb in Gleevec groups; there was no significant difference between H2O and Gleevec in anti-NK1.1 mAb groups).
Figure 3
Gleevec alone or combined with FL induced NK cell activation in vivo. (A) Long-term exposure to Gleevec in C57BL/6 mice induced reduction of the splenic T lymphocyte counts but selectively maintained the NK cell subset. After red blood cell removal and an adherence step, splenocytes were enumerated after 15-–21 days of oral feeding with Gleevec (150 mg/kg bid) or H2O (200 μl) and analyzed by flow cytometry using anti-CD3 and anti-NK1.1 mAb’s. The absolute numbers of CD3+/NK1.1– T cells and CD3–/NK1.1+ NK cells were deduced from the percentages obtained in 2 independent experiments and are indicated in the boxes (B). T lymphocytes were not activated during Gleevec oral feeding. In the CD3+/NK1.1– T cell gate, the CD69 expression is shown. (C) NK lymphocytes were activated during therapy with Gleevec. In the CD3–/NK1.1+ NK cell gate, the CD69 expression is shown. Swiss_nu/nu_ mice (D) and C57BL/6 littermates (E) were injected intraperitonealy with 10 μg of FL or 100 μl of PBS each day for 10 days. From day 7 to day 10, mice received either Gleevec (150 mg/kg bid) or H2O (200 μl). At day 11, all mice were sacrificed to analyze the expression of the NK activation marker CD69 on NK1.1+ or DX5+/CD3– splenocytes. Positive controls included mice treated with rhuIL-2 (1 × 105 IU intraperitoneally, bid for 4 days). Groups were compared by analysis of variance (ANOVA) using the nonparametric Kruskall-Wallis test. *P < 0.05 as compared to PBS. #P < 0.05 as compared to Gleevec. P < 0.05 as compared to FL.
Figure 4
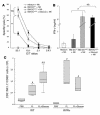
Gleevec endowed DCs with NK cell stimulatory capacity. (A) Mouse DCs pretreated with Gleevec exhibited enhanced NK cell stimulatory capacity in vitro. BM-DC+NK coculture supernatants were monitored for IFN-γ secretion. Gleevec alone or FL+Gleevec did not trigger NK cell cytotoxicity or IFN-γ production in the absence of BM-DCs. (B) Gleevec but not tyrphostin (AG957) enhanced the NK stimulatory activity of DCs. Experiments were conducted in triplicate at various DC/NK ratios (1:2, 1:10) in the presence of Gleevec or tyrphostin. (C) IL-12 is not involved in the Gleevec-mediated NK cell activation. Conventions as in A but using IL-12p35 loss-of-function BM-DCs instead of WT BM-DCs. (D) STI-mediated NK cell activation depends on cell-cell contact. BM-DC and NK cell cocultures were separated or not by a trans-well membrane (BM-DC // NK). (E) Long-term exposure to Gleevec enhanced the host CD11c+ DC capacity to activate NK cells. Cell-sorted CD11c+ B220– splenocytes from C57BL/6 mice treated either with H2O or Gleevec for 15–21 days were incubated for 20 hours with NK cells as in A. IFN-γ release was measured. One representative experiment (out of 2) is shown. (F) Human CD34+-derived DCs stimulated with Gleevec also promoted NK cell activation. After coculture of CD34+-derived DCs with purified human NK cells, NK cell cytolytic activity was observed against K562. Means of triplicate wells are represented (standard errors were consistently less than 10% of means). One representative experiment (out of 5) is depicted. Groups were compared by ANOVA using the nonparametric Kruskall-Wallis test (*P < 0.05).
Figure 5
The c-kit loss-of-function mutation W/Wv conferred a phenotype similar, with regard to DC-mediated NK cell activation, to that found with Gleevec treatment. (A) Deficient KIT signaling enhanced the capacity of DCs to stimulate the cytotoxic activity of NK cells in vitro. The experimental setting was identical to that represented in Figure 4A, except that BM-DCs derived from WT WBB6F1 mice (WT) or from c-kit-deficient WBB6F1 mice (W/Wv) were cocultured with WT NK cells and NK cytotoxicity was assessed on YAC-1 cells (12). (B) Deficient KIT signaling stimulated the capacity of DCs to elicit IFN-γ secretion by NK cells in vitro. Instead of measuring the cytotoxic activity as in A, the accumulation of IFN-γ in culture supernatants was assessed. (C) The W/Wv mutation allowed FL-mediated NK cell activation in vivo. WT and- W/Wv mutant mice were treated with FL in vivo (same doses, schedule, and statistical methods as in Figure 3). All experiments were performed 3 times with similar results. #P < 0.05, significantly different from PBS-treated animals (in W/Wv and WT animals); *P < 0.05, significantly different from FL-treated animals.
Figure 6
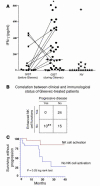
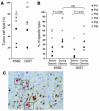
Correlation between NK cell activation and disease control in GIST patients. (A) Therapy with Gleevec-induced enhanced NK cell IFN-γ production. Blood NK cells were purified from GIST patients (Supplemental Table 1) at diagnosis after 2–12 months of therapy with 400 mg of Gleevec or from sex- and age-matched normal volunteers (NV) and cocultured with MD-DCs in the presence of LPS as described in Methods. Filled symbols correspond to individual patients or controls. Circles, objective responses; triangles; progressive disease. A longitudinal study enrolling 11 cases before and after Gleevec treatment is depicted with lines. Asterisk indicates significant differences between mean values of IFN-γ production after Gleevec compared with prior Gleevec or controls (P < 0.05) in 37 consecutive patients. (B) Correlation between clinical outcome and NK cell activation induced by therapy with Gleevec. The clinical responders exhibited stable disease, or partial or complete regressions (WHO criteria). The biological responders exhibited NK cell IFN-γ secretion above 130 pg/ml after at least 2 months of Gleevec therapy (cut-off defined according to the data shown in A). A significant correlation between NK cell activation and clinical outcome at the time of NK cell activation assessment was found (**P = 0.03, Fisher’s exact method). (C) NK cell activation is associated with prolonged time to progression in GIST patients treated with Gleevec. The study of the time to progression was performed for 43 patients with a median follow-up of 13.2 months. Patients who exhibited enhanced NK cell functions at 2 months of Gleevec (n = 22; red) and those who did not (n = 21; blue) (log rank test, P = 0.03).
Figure 7
GISTs are NK cell–sensitive targets. (A) NK cell recognition of a GIST cell line. CD3–/CD56+ NK cells from GIST patients at diagnosis were activated overnight with 1000 IU rhuIL-2. The cytolytic activity of these NK cells was tested against the NK cell-–sensitive K562 targets and against a GIST cell line in a 51Cr release assay, using an E/T ratio of 10:1. Data represent the means of triplicate wells of 7 different GIST patients. Each symbol represents an individual patient’s NK cell lysis of both targets (see key in B). (B) Gleevec promoted enhanced NK cell recognition of GIST cells. Experimental settings were the same as in A, but cytotoxicity assays had been performed before and 2 months after initiation of Gleevec therapy. (C) DC/NK cell cross-talk in a Gleevec-induced lichenoid dermatitis. Skin biopsies from a lichenoid dermatitis were taken from a patient bearing GISTs in complete regression after a year of oral administration of Gleevec. Formol-fixed and paraffin-embedded sections (4 μm thick) were immunohistochemically stained with an anti-DC-LAMP mAb (Schering-Plough Corp., Dardilly, France) and anti-CD57 mAb (NK1, Dako A/S, Glostrup, Denmark). Double-staining with anti-CD3 and anti-CD57 mAb demonstrated that CD57+ cells were all CD3–. DC-LAMP+ mature dendritic cells were visualized by light microscope (brown staining). CD57+ NK cells (3 black boxes) were identified by their red color and visible nuclei (×400 magnification).
Similar articles
- Imatinib mesylate: in the treatment of gastrointestinal stromal tumours.
Croom KF, Perry CM. Croom KF, et al. Drugs. 2003;63(5):513-22; discussion 523-4. doi: 10.2165/00003495-200363050-00005. Drugs. 2003. PMID: 12600228 Review. - Targeting c-kit mutations in solid tumors: scientific rationale and novel therapeutic options.
Demetri GD. Demetri GD. Semin Oncol. 2001 Oct;28(5 Suppl 17):19-26. Semin Oncol. 2001. PMID: 11740803 Review. - Effect of imatinib mesylate on neuroblastoma tumorigenesis and vascular endothelial growth factor expression.
Beppu K, Jaboine J, Merchant MS, Mackall CL, Thiele CJ. Beppu K, et al. J Natl Cancer Inst. 2004 Jan 7;96(1):46-55. doi: 10.1093/jnci/djh004. J Natl Cancer Inst. 2004. PMID: 14709738 - Gastrointestinal stromal tumors: overview of pathologic features, molecular biology, and therapy with imatinib mesylate.
Koh JS, Trent J, Chen L, El-Naggar A, Hunt K, Pollock R, Zhang W. Koh JS, et al. Histol Histopathol. 2004 Apr;19(2):565-74. doi: 10.14670/HH-19.565. Histol Histopathol. 2004. PMID: 15024716 Review.
Cited by
- Strategies for Potentiating NK-Mediated Neuroblastoma Surveillance in Autologous or HLA-Haploidentical Hematopoietic Stem Cell Transplants.
Bottino C, Della Chiesa M, Sorrentino S, Morini M, Vitale C, Dondero A, Tondo A, Conte M, Garaventa A, Castriconi R. Bottino C, et al. Cancers (Basel). 2022 Sep 20;14(19):4548. doi: 10.3390/cancers14194548. Cancers (Basel). 2022. PMID: 36230485 Free PMC article. Review. - ABCC3 Expressed by CD56dim CD16+ NK Cells Predicts Response in Glioblastoma Patients Treated with Combined Chemotherapy and Dendritic Cell Immunotherapy.
Pellegatta S, Di Ianni N, Pessina S, Paterra R, Anghileri E, Eoli M, Finocchiaro G. Pellegatta S, et al. Int J Mol Sci. 2019 Nov 23;20(23):5886. doi: 10.3390/ijms20235886. Int J Mol Sci. 2019. PMID: 31771235 Free PMC article. - Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido.
Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, Sorenson EC, Popow R, Ariyan C, Rossi F, Besmer P, Guo T, Antonescu CR, Taguchi T, Yuan J, Wolchok JD, Allison JP, DeMatteo RP. Balachandran VP, et al. Nat Med. 2011 Aug 28;17(9):1094-100. doi: 10.1038/nm.2438. Nat Med. 2011. PMID: 21873989 Free PMC article. - VEGFA/VEGFR2-targeted therapies prevent the VEGFA-induced proliferation of regulatory T cells in cancer.
Terme M, Tartour E, Taieb J. Terme M, et al. Oncoimmunology. 2013 Aug 1;2(8):e25156. doi: 10.4161/onci.25156. Epub 2013 Jun 10. Oncoimmunology. 2013. PMID: 24083078 Free PMC article. - Cancer-Induced Alterations of NK-Mediated Target Recognition: Current and Investigational Pharmacological Strategies Aiming at Restoring NK-Mediated Anti-Tumor Activity.
Chretien AS, Le Roy A, Vey N, Prebet T, Blaise D, Fauriat C, Olive D. Chretien AS, et al. Front Immunol. 2014 Mar 24;5:122. doi: 10.3389/fimmu.2014.00122. eCollection 2014. Front Immunol. 2014. PMID: 24715892 Free PMC article. Review.
References
- Rubin BP, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–8121. - PubMed
- Heinrich MC, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;31:708–710. - PubMed
- Apperley JF, et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N. Engl. J. Med. 2002;347:481–487. - PubMed
- Buchdunger E, O’Reilly T, Wood J. Pharmacology of imatinib (STI571) Eur. J. Cancer. 2002;38:S28–S36. - PubMed
- Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J. Clin. Oncol. 2002;20:1692–1703. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources