Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis - PubMed (original) (raw)
Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis
Roderick J Phillips et al. J Clin Invest. 2004 Aug.
Abstract
Previous reports have identified a circulating pool of CD45(+) collagen I(+) CXCR4(+) (CD45(+)Col I(+)CXCR4(+)) cells, termed fibrocytes, that traffic to areas of fibrosis. No studies have demonstrated that these cells actually contribute to fibrosis, however. Pulmonary fibrosis was originally thought to be mediated solely by resident lung fibroblasts. Here we show that a population of human CD45(+)Col I(+)CXCR4(+) circulating fibrocytes migrates in response to CXCL12 and traffics to the lungs in a murine model of bleomycin-induced pulmonary fibrosis. Next, we demonstrated that murine CD45(+)Col I(+)CXCR4(+) fibrocytes also traffic to the lungs in response to a bleomycin challenge. Maximal intrapulmonary recruitment of CD45(+)Col I(+)CXCR4(+) fibrocytes directly correlated with increased collagen deposition in the lungs. Treatment of bleomycin-exposed animals with specific neutralizing anti-CXCL12 Ab's inhibited intrapulmonary recruitment of CD45(+)Col I(+)CXCR4(+) circulating fibrocytes and attenuated lung fibrosis. Thus, our results demonstrate, we believe for the first time, that circulating fibrocytes contribute to the pathogenesis of pulmonary fibrosis.
Figures
Figure 1
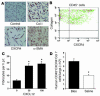
Characterization of human fibrocytes and their trafficking to bleomycin-induced lung fibrosis in SCID mice. (A) Human fibrocytes stained with control Ab, human Col I, human CXCR4, and human α-SMA 3 weeks after purification. Representative fields viewed at ×400 magnification. (B) Isolated human fibrocytes triple-stained for Col I, CD45, and CXCR4 were examined by FACS analysis. Data shows CD45+ fibrocytes that were examined for dual expression of CXCR4 and Col I. (C) Isolated human fibrocyte chemotaxis in response to 0, 30, and 100 ng/ml of CXCL12. Data are representative of three experiments. *P < 0.05. h.p.f., high-powered field. (D) Isolated human fibrocytes (106) were injected into the tail vein of SCID mice at day 4 after treatment with either intratracheal bleomycin (Bleo) or saline, followed by recovery of cells from the lungs at day 8. Cells were then stained for human CD45, Col I, and CXCR4 and analyzed by flow cytometry to determine trafficking of human fibrocytes to the lungs of SCID mice. *P < 0.05.
Figure 2
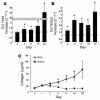
Kinetics of Col I and Col III gene expression and total collagen protein deposition in the lung during bleomycin-induced pulmonary fibrosis. (A and B) Kinetics of pro-Col I (A) and pro–Col III (B) gene expression in lungs of mice exposed to intratracheal bleomycin compared with saline control as determined by real-time quantitative PCR. n = 6 lungs in each group. Data represent the mean ± SEM. *P < 0.05, significant differences between bleomycin and saline groups. ct, threshold cycle number. (C) Kinetics of total collagen protein deposition in lungs of mice exposed to either intratracheal bleomycin, saline, or naive (time 0) control as determined by the Sircol assay. n = 6 lungs in each group. Data represent the mean ± SEM. *P < 0.05, significant differences between bleomycin and saline groups.
Figure 3
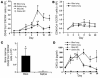
Intrapulmonary recruitment of CD45+Col I+CXCR4+ fibrocytes is greater than CD45+Col I+CCR7+ fibrocytes and correlates with collagen deposition in the lungs of bleomycin-exposed mice. (A and B) Single-cell suspensions were isolated from bleomycin- or saline-challenged lungs and blood buffy coats at the times indicated, triple-stained for CD45, Col I, and CXCR4 (A), or CD45, Col I, and CCR7 (lungs only) (B), and then examined by FACS analysis. n = 6 samples per group. BC, buffy coat. Data represent the mean ± SEM. *P < 0.05, significant differences between bleomycin and saline groups. **P < 0.05, significant differences between saline-exposed mice and the naive mice. (C) Mice were treated with either intratracheal bleomycin or saline for 8 days. Bone marrow was removed, triple-stained for CD45, Col I, and CXCR4, and then examined by FACS analysis. n = 3 samples per group. Data represent the mean ± SEM. *P < 0.05. (D) Kinetics of CXCL12 protein expression in lung tissue and plasma of mice exposed to either intratracheal bleomycin, saline, or naive control (day 0) as determined by ELISA. n = 6 samples in each group. Data represents the mean ± SEM. *P < 0.05, significant differences between bleomycin and saline groups. **P < 0.05, significant differences between saline-exposed mice and the naive mice.
Figure 4
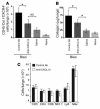
Neutralizing anti-CXCL12 Ab’s inhibit intrapulmonary recruitment of CD45+Col I+CXCR4+ fibrocytes and significantly attenuate lung fibrosis in bleomycin-treated mice. (A) Single-cell suspensions were isolated from the lungs of naive or saline- or bleomycin-exposed mice, where the bleomycin-treated mice also received daily injections of either anti-CXCL12 Ab or control Ab for 16 days. These cells were then triple-stained for CD45, Col I, and CXCR4 and analyzed by flow cytometry. n = 5 samples per group. Data represent the mean ± SEM. *P < 0.05. (B) Total collagen present in lung tissue of naive, saline-, or bleomycin-exposed mice, where the bleomycin-treated mice also received daily injections of either anti-CXCL12 or control Ab’s for 16 days. Total collagen levels were determined by the Sircol assay. n = 4 lungs in each group. Data represent the mean ± SEM. *P < 0.05. (C) Single-cell suspensions were isolated from the lungs of bleomycin-exposed mice treated with either daily injections of anti-CXCL12 or control Ab’s for 8 days. These cells were then individually stained for CD3, CD4, CD8, NK1.1, Ly6, and Mac519 (Mac) and analyzed by flow cytometry. n = 3 lungs per group.
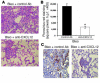
Figure 5
Representative histopathology and morphometric analysis of picrosirius red in bleomycin-induced pulmonary fibrosis and expression of α-SMA in the presence of neutralizing anti-CXCL12 or control Ab’s. (A) Representative H&E-stained histopathologic sections of lung tissue on day 16 after intratracheal bleomycin administration in the presence of daily injections of either control (upper panel) or neutralizing anti-CXCL12 Ab’s (lower panel). (B) Morphometric analysis of picrosirius red–stained lung tissue was measured by image analysis (NIH Image 1.55) and expressed as area (square pixels) at ×400 magnification. *P < 0.001. (C) Histopathologic sections of lung tissue on day 16 after intratracheal bleomycin administration in the presence of daily injections of either control (left panel) or neutralizing anti-CXCL12 Ab’s (right panel) stained with α-SMA. Representative fields viewed at ×400 magnification.
Comment in
- Pulmonary fibrosis: thinking outside of the lung.
Garantziotis S, Steele MP, Schwartz DA. Garantziotis S, et al. J Clin Invest. 2004 Aug;114(3):319-21. doi: 10.1172/JCI22497. J Clin Invest. 2004. PMID: 15286797 Free PMC article. Review.
Similar articles
- Fibrocytes contribute to inflammation and fibrosis in chronic hypersensitivity pneumonitis through paracrine effects.
García de Alba C, Buendia-Roldán I, Salgado A, Becerril C, Ramírez R, González Y, Checa M, Navarro C, Ruiz V, Pardo A, Selman M. García de Alba C, et al. Am J Respir Crit Care Med. 2015 Feb 15;191(4):427-36. doi: 10.1164/rccm.201407-1334OC. Am J Respir Crit Care Med. 2015. PMID: 25531246 - Bone marrow-derived progenitor cells in pulmonary fibrosis.
Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Hashimoto N, et al. J Clin Invest. 2004 Jan;113(2):243-52. doi: 10.1172/JCI18847. J Clin Invest. 2004. PMID: 14722616 Free PMC article. - Antifibrotic effects of CXCR4 antagonist in bleomycin-induced pulmonary fibrosis in mice.
Makino H, Aono Y, Azuma M, Kishi M, Yokota Y, Kinoshita K, Takezaki A, Kishi J, Kawano H, Ogawa H, Uehara H, Izumi K, Sone S, Nishioka Y. Makino H, et al. J Med Invest. 2013;60(1-2):127-37. doi: 10.2152/jmi.60.127. J Med Invest. 2013. PMID: 23614921 - The extrapulmonary origin of fibroblasts: stem/progenitor cells and beyond.
Lama VN, Phan SH. Lama VN, et al. Proc Am Thorac Soc. 2006 Jun;3(4):373-6. doi: 10.1513/pats.200512-133TK. Proc Am Thorac Soc. 2006. PMID: 16738203 Free PMC article. Review. - The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis.
Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B. Strieter RM, et al. J Leukoc Biol. 2009 Nov;86(5):1111-8. doi: 10.1189/jlb.0309132. Epub 2009 Jul 6. J Leukoc Biol. 2009. PMID: 19581373 Free PMC article. Review.
Cited by
- Targeting nanoparticles to lung cancer-derived A549 cells based on changes on interstitial stiffness in biomimetic models.
Kohon AI, Man K, Hessami A, Mathis K, Webb J, Fang J, Radfar P, Yang Y, Meckes B. Kohon AI, et al. iScience. 2024 Sep 23;27(10):111015. doi: 10.1016/j.isci.2024.111015. eCollection 2024 Oct 18. iScience. 2024. PMID: 39435151 Free PMC article. - Single-Cell RNA-Sequencing Identifies Bone Marrow-Derived Progenitor Cells as a Main Source of Extracellular Matrix-Producing Cells Across Multiple Organ-Based Fibrotic Diseases.
Zhong Y, Wei B, Wang W, Chen J, Wu W, Liang L, Huang XR, Szeto CC, Yu X, Nikolic-Paterson DJ, Lan HY. Zhong Y, et al. Int J Biol Sci. 2024 Sep 16;20(13):5027-5042. doi: 10.7150/ijbs.98839. eCollection 2024. Int J Biol Sci. 2024. PMID: 39430238 Free PMC article. - Understanding myofibroblast origin in the fibrotic lung.
Zabihi M, Shahriari Felordi M, Lingampally A, Bellusci S, Chu X, El Agha E. Zabihi M, et al. Chin Med J Pulm Crit Care Med. 2024 Sep 17;2(3):142-150. doi: 10.1016/j.pccm.2024.08.003. eCollection 2024 Sep. Chin Med J Pulm Crit Care Med. 2024. PMID: 39403408 Free PMC article. Review. - Pravastatin prevents colitis-associated carcinogenesis by reducing CX3CR1high M2-like fibrocyte counts in the inflamed colon.
Hachiya K, Masuya M, Kuroda N, Yoneda M, Nishimura K, Shiotani T, Tawara I, Katayama N. Hachiya K, et al. Sci Rep. 2024 Oct 3;14(1):23021. doi: 10.1038/s41598-024-74215-9. Sci Rep. 2024. PMID: 39362935 Free PMC article. - Lung fibrosis in sarcoidosis. Is there a place for antifibrotics?
Bączek K, Piotrowski WJ. Bączek K, et al. Front Pharmacol. 2024 Aug 30;15:1445923. doi: 10.3389/fphar.2024.1445923. eCollection 2024. Front Pharmacol. 2024. PMID: 39281278 Free PMC article. Review.
References
- Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am. J. Respir. Crit. Care Med. 1994;150:967–972. - PubMed
- Perez A, Rogers RM, Dauber JH. The prognosis of idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2003;29(3 Suppl.):S19–S26. - PubMed
- du Bois R. Diffuse lung disease: a view for the future. Sarcoidosis Vasc. Diffuse Lung Dis. 1997;14:23–30. - PubMed
- Dacic S, Yousem SA. Histologic classification of idiopathic chronic interstitial pneumonias. Am. J. Respir. Cell Mol. Biol. 2003;29(3 Suppl.):S5–S9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL-03906/HL/NHLBI NIH HHS/United States
- P50 HL067665/HL/NHLBI NIH HHS/United States
- HL-66027/HL/NHLBI NIH HHS/United States
- P50HL-67665/HL/NHLBI NIH HHS/United States
- R01 HL066027/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous