Primitive neural stem cells from the mammalian epiblast differentiate to definitive neural stem cells under the control of Notch signaling - PubMed (original) (raw)
Comparative Study
. 2004 Aug 1;18(15):1806-11.
doi: 10.1101/gad.1208404.
Affiliations
- PMID: 15289455
- PMCID: PMC517401
- DOI: 10.1101/gad.1208404
Comparative Study
Primitive neural stem cells from the mammalian epiblast differentiate to definitive neural stem cells under the control of Notch signaling
Seiji Hitoshi et al. Genes Dev. 2004.
Abstract
Basic fibroblast growth factor (FGF2)-responsive definitive neural stem cells first appear in embryonic day 8.5 (E8.5) mouse embryos, but not in earlier embryos, although neural tissue exists at E7.5. Here, we demonstrate that leukemia inhibitory factor-dependent (but not FGF2-dependent) sphere-forming cells are present in the earlier (E5.5-E7.5) mouse embryo. The resultant clonal sphere cells possess self-renewal capacity and neural multipotentiality, cardinal features of the neural stem cell. However, they also retain some nonneural properties, suggesting that they are the in vivo cells' equivalent of the primitive neural stem cells that form in vitro from embryonic stem cells. The generation of the in vivo primitive neural stem cell was independent of Notch signaling, but the activation of the Notch pathway was important for the transition from the primitive to full definitive neural stem cell properties and for the maintenance of the definitive neural stem cell state.
Figures
Figure 1.
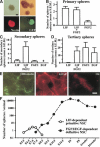
LIF-dependent primitive neural stem cell spheres. (A) The spheres (top left) generated from the E7.5 mouse neuroectoderm were immunopositive for nestin (bottom left). Dissociated E7.5 neuroectoderm cells from CD1 embryos and from GFP mouse embryos were mixed in equivalent proportions (to a final cell density of 10 cells/μL) and proliferated to form LIF-dependent spheres (top right). The complete lack of GFP+ cells in the white spheres (bottom right) shows that the spheres were clonally derived from single cells. Bar, 0.1 mm. (B) The average sphere numbers in the presence of LIF (n = 32 embryos), FGF2 (n = 16 embryos), or both (n = 35 embryos) per embryo are shown. (C) Single primary LIF-dependent spheres produced secondary spheres in the presence of both LIF and FGF2 (n = 10 or more). (D) Single secondary spheres cultured in both LIF and FGF2 responded to either FGF2 or EGF to produce tertiary spheres (n = 16 or more). (E) The primary and passaged E7.5 sphere contained cells that could differentiate into βIII tubulin+ neurons, GFAP+ astrocytes, or O4+ oligodendrocytes in vitro. Bar, 40 μm. (F) The average numbers of LIF-dependent spheres (open circles) and FGF2/EGF-dependent forebrain neurospheres (closed circles) per animal that were isolated at different times throughout development and into the adult mouse are plotted.
Figure 2.
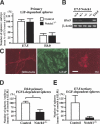
Gene expression profiles of the primary tissue and clonal spheres. (A) Primary E7.5 neuroectoderm-derived spheres (E7.5S), tissue (E7.5T) from which the sphere derived, and E14.5 definitive neural stem cell neurospheres (E14.5S) were analyzed by RT–PCR for the expression of FGF2 and FGF receptor 1 (FGFR1). (B,C) Primary and tertiary E6.5 epiblast-derived spheres (1° and 3° E6.5), as well as ES-derived primitive neural stem cell spheres (1° and 3° ES) and primary E14.5 definitive neural stem cell spheres (E14.5) were analyzed by RT–PCR. (D) The amounts of Hes5 gene mRNA were quantified by real time RT–PCR using the LightCycler system. The ratios of Hes5 mRNA copy numbers to those of β_-actin_ are shown. Data represent means ± S.E.M. (*) P < 0.05.
Figure 3.
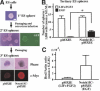
The formation of neural stem cell spheres from Notch1 mutant embryos. (A) The numbers of primary LIF-dependent spheres from E7.5 and E8.0 _Notch1_-/- embryos (n = 7 at E7.5 and n = 6 at E8.0) and littermate controls (n = 18 at E7.5 and n = 25 at E8.0) are shown. (B) The expression of Hes5 was abolished in the primary E7.5 _Notch1_-/- spheres. (C) The primary E7.5 _Notch1_-/- spheres retained multipotentiality within the neural lineage. Bar, 50 μm. (D) The numbers of primary FGF2-dependent neurospheres from E8.0_Notch1_-/- embryos (n = 19) and littermate controls (n = 57). (*) P < 0.05. (E) The primary LIF-dependent spheres from each E7.5_Notch1_-/- (n = 7) and littermate control (n = 18) embryo were separately passaged twice in vitro to generate tertiary EGF-dependent neurospheres. The numbers of EGF-dependent tertiary neurospheres from each embryo are shown.
Figure 4.
Active Notch1 retrovirus infection of ES spheres. (A) A schema of the experimental procedures. Some of secondary spheres infected with the retrovirus showed GFP+expression. After passaging, tertiary spheres infected with pMXIE-Notch1IC were homogenously positive for c-Myc that is tagged to Notch1IC. Bar, 0.1 mm. (B) The numbers of tertiary spheres generated in both LIF and FGF2 and in EGF-only media are shown. Data represent means ± S.E.M. from three independent experiments. (*) P < 0.05. (C) The amounts of_Hes5_ gene expression were quantified (n = 3).
Figure 5.
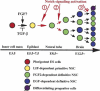
A model of neural stem cell development. LIF-dependent primitive neural stem cells can be generated directly from single ES cell in vitro after relieving TGF-β inhibition (Tropepe et al. 2001) and now have been shown to exist (at least potentially in vivo and literally in vitro) in the E5.5–E7.5 epiblast/neuroectoderm of mouse embryos. Whether or not FGF signaling promotes the induction of primitive neural stem cells in vivo (or just their survival or proliferation) remains to be determined. Activation of the Notch pathway could be required for the transition from primitive to definitive neural stem cells that autonomously acquire EGF responsiveness ( ) and/or for the maintenance of definitive, FGF2- or EGF-dependent neural stem cells by enhancing their self-renewal (
) and/or for the maintenance of definitive, FGF2- or EGF-dependent neural stem cells by enhancing their self-renewal ( ) and thus suppressing their differentiation into neuronal or glial unipotential progenitors (
) and thus suppressing their differentiation into neuronal or glial unipotential progenitors ( ).
).
Similar articles
- [The generation of neural stem cells: induction of neural stem cells from embryonic stem (ES) cells].
Hitoshi S. Hitoshi S. Rinsho Shinkeigaku. 2003 Nov;43(11):827-9. Rinsho Shinkeigaku. 2003. PMID: 15152476 Review. Japanese. - Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells.
Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Hitoshi S, et al. Genes Dev. 2002 Apr 1;16(7):846-58. doi: 10.1101/gad.975202. Genes Dev. 2002. PMID: 11937492 Free PMC article. - Effect of leukemia inhibitory factor on embryonic stem cell differentiation: implications for supporting neuronal differentiation.
He Z, Li JJ, Zhen CH, Feng LY, Ding XY. He Z, et al. Acta Pharmacol Sin. 2006 Jan;27(1):80-90. doi: 10.1111/j.1745-7254.2006.00254.x. Acta Pharmacol Sin. 2006. PMID: 16364214 - Induction of a high population of neural stem cells with anterior neuroectoderm characters from epiblast-like P19 embryonic carcinoma cells.
Xia C, Wang C, Zhang K, Qian C, Jing N. Xia C, et al. Differentiation. 2007 Dec;75(10):912-27. doi: 10.1111/j.1432-0436.2007.00188.x. Epub 2007 Jun 16. Differentiation. 2007. PMID: 17573917 - Dynamic regulation of Notch signaling in neural progenitor cells.
Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Kageyama R, et al. Curr Opin Cell Biol. 2009 Dec;21(6):733-40. doi: 10.1016/j.ceb.2009.08.009. Epub 2009 Sep 23. Curr Opin Cell Biol. 2009. PMID: 19783418 Review.
Cited by
- Selective roles of normal and mutant huntingtin in neural induction and early neurogenesis.
Nguyen GD, Gokhan S, Molero AE, Mehler MF. Nguyen GD, et al. PLoS One. 2013 May 14;8(5):e64368. doi: 10.1371/journal.pone.0064368. Print 2013. PLoS One. 2013. PMID: 23691206 Free PMC article. - Epigenetic control on cell fate choice in neural stem cells.
Hu XL, Wang Y, Shen Q. Hu XL, et al. Protein Cell. 2012 Apr;3(4):278-90. doi: 10.1007/s13238-012-2916-6. Epub 2012 May 2. Protein Cell. 2012. PMID: 22549586 Free PMC article. Review. - TIN2 deficiency leads to ALT-associated phenotypes and differentiation defects in embryonic stem cells.
Yin S, Zhang F, Lin S, Chen W, Weng K, Liu D, Wang C, He Z, Chen Y, Ma W, Huang J, Huang Y, Songyang Z. Yin S, et al. Stem Cell Reports. 2022 May 10;17(5):1183-1197. doi: 10.1016/j.stemcr.2022.03.005. Epub 2022 Apr 7. Stem Cell Reports. 2022. PMID: 35395177 Free PMC article. - Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences.
Smukler SR, Runciman SB, Xu S, van der Kooy D. Smukler SR, et al. J Cell Biol. 2006 Jan 2;172(1):79-90. doi: 10.1083/jcb.200508085. J Cell Biol. 2006. PMID: 16390999 Free PMC article. - The responses of neural stem cells to the level of GSK-3 depend on the tissue of origin.
Holowacz T, Alexson TO, Coles BL, Doble BW, Kelly KF, Woodgett JR, Van Der Kooy D. Holowacz T, et al. Biol Open. 2013 Jun 20;2(8):812-21. doi: 10.1242/bio.20131941. eCollection 2013 Aug 15. Biol Open. 2013. PMID: 23951407 Free PMC article.
References
- Artavanis-Tsakonas S., Matsuno, K., and Fortini, M.E.1995. . Notch signaling. Science 268:225 -232. - PubMed
- Conlon R.A., Reaume, A.G., and Rossant, J.1995. . Notch1 is required for the coordinate segmentation of somites. Development 121:1533 -1545. - PubMed
- de la Pompa J.L., Wakeham, A., Correia, K.M., Samper, E., Brown, S., Aguilera, R.J., Nakano, T., Honjo, T., Mak, T.W., Rossant, J., et al. 1997. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 124:1139 -1148. - PubMed
- Doetsch F., Caille, I., Lim, D.A., Garcia-Verdugo, J.M., and Alvarez-Buylla, A.1999. . Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97:703 -716. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical