Effect of nanomolar concentrations of sodium dodecyl sulfate, a catalytic inductor of alpha-helices, on human calcitonin incorporation and channel formation in planar lipid membranes - PubMed (original) (raw)
Effect of nanomolar concentrations of sodium dodecyl sulfate, a catalytic inductor of alpha-helices, on human calcitonin incorporation and channel formation in planar lipid membranes
Silvia Micelli et al. Biophys J. 2004 Aug.
Abstract
Human Calcitonin (hCt) is a peptide hormone which has a regulatory action in calcium-phosphorus metabolism. It is currently used as a therapeutic tool in bone pathologies such as osteoporosis and Paget's disease. However, due to its amphiphilic property tends to form a gelatinous solution in water which consists of fibrils that limits its therapeutic use. Here we show that sodium dodecyl sulfate (SDS), an anionic detergent able to induce and stabilize alpha-helices in polypeptides, at a monomeric concentration ranging between 0.26 mM-5 pM (all concentrations are below the CMC), increases the rate and number of hCt channel formation in planar lipid membranes, at both high and low hCt concentrations, with a maximum increase at a molecular hCt/SDS ratio of 1000:1. This effect could be interpreted as a counteraction to the fibrillation process of hCt molecules by removing molecules available for aggregation from the fluid; furthermore, this action, independently of channel formation in the cell membrane, could improve the peptide-receptor interaction. The action of SDS could be attributable to the strength of the sulfate negative charge and the hydrophobic chain; in fact, a similar effect was obtained with lauryl sarcosine and not with a neutral detergent such as n-dodecyl-beta-D-maltoside. The very low molecular ratio between SDS and peptide is suggestive of a possible catalytic action of SDS that could induce alpha-helices, the appropriate structures for interacting with the membrane. Moreover, in the experimental conditions investigated, the addition of SDS does not modify the membrane's electrical properties and most of the channel properties. This finding may contribute to the knowledge of environment-folding diseases due to protein and peptides.
Figures
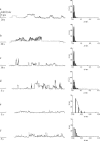
FIGURE 1
Single-channel features of hCt in the absence and in the presence of SDS with associated histograms of the conductance fluctuations. (a) hCt 125 nM; (b) hCt 125 nM + SDS (0.26 mM); (c) hCt 24.5 nM; (d) hCt 24.5 nM + SDS (0.26 mM); (e) hCt 5 nM; and (f) hCt 5 nM + SDS (5 pM). Experiments were performed on a POPC/DOPG (85:15) membrane in the presence of hCt and of hCt + SDS added to the _cis_-side; the voltage was set to +150 mV, the aqueous phase contained 1M KCl (pH 7), and T = 22°C. Note the increase in channel occurrence (channels/minute) when SDS was present in the medium.
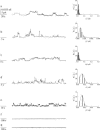
FIGURE 2
Single-channel features of hCt in the absence and in the presence of SDS at different concentrations with associated histograms of the conductance fluctuations. (a) hCt 125 nM; (b) hCt 125 nM + SDS (12.5 nM); (c) hCt 125 nM + SDS (1.25 nM); (d) hCt 125 nM + SDS (0.125 nM); (e) hCt 125 nM + SDS (0.0125 nM); (f) SDS (0.26 mM); and (g) SDS (0.125 nM). Experiments were performed on a POPC/DOPG (85:15) membrane in the presence of hCt and of hCt + SDS added to the _cis_-side; the voltage was set to +150 mV, the aqueous phase contained 1M KCl (pH 7), and T = 22°C.
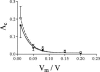
FIGURE 3
Conductance-voltage relationship for hCt channels in the absence (▪) or presence of SDS (□). Experimental conditions: KCl 1M, hCt (49 nM) or hCt (49 nM) + SDS (0.26 mM) was present on the cis sides of the POPC/DOPG (85:15) membrane. The curves superimposed on the data are the results of the fit with the model: _λ_c = Ae(−KVm) + p, where A is the difference between the conductance at _V_m = 0 and at _V_m = membrane black (p); K is the constant correlated with the gating charge n (n = KRT/F). (▪) A = 0.234 ±0.004 (nS); p = 0.0067 (nS); K = 37.13 ± 0.59 (_V_−1); _R_2 = 0.988. (□) A = 0.29 ± 0.002 (nS); p = 0.0067 (nS); K = 38.3 ± 0.48(_V_−1); _R_2= 0.99.
Similar articles
- Acetyl-[Asn30,Tyr32]-calcitonin fragment 8-32 forms channels in phospholipid planar lipid membranes.
Meleleo D, Gallucci E, Picciarelli V, Micelli S. Meleleo D, et al. Eur Biophys J. 2007 Sep;36(7):763-70. doi: 10.1007/s00249-007-0150-6. Epub 2007 Mar 29. Eur Biophys J. 2007. PMID: 17393160 - Magainin 2 channel formation in planar lipid membranes: the role of lipid polar groups and ergosterol.
Gallucci E, Meleleo D, Micelli S, Picciarelli V. Gallucci E, et al. Eur Biophys J. 2003 Mar;32(1):22-32. doi: 10.1007/s00249-002-0262-y. Epub 2002 Nov 22. Eur Biophys J. 2003. PMID: 12632203 - Effects of phenylalanine substitutions in gramicidin A on the kinetics of channel formation in vesicles and channel structure in SDS micelles.
Jordan JB, Easton PL, Hinton JF. Jordan JB, et al. Biophys J. 2005 Jan;88(1):224-34. doi: 10.1529/biophysj.104.047456. Epub 2004 Oct 22. Biophys J. 2005. PMID: 15501932 Free PMC article. - Ion channel formation and membrane-linked pathologies of misfolded hydrophobic proteins: the role of dangerous unchaperoned molecules.
Kourie JI, Henry CL. Kourie JI, et al. Clin Exp Pharmacol Physiol. 2002 Sep;29(9):741-53. doi: 10.1046/j.1440-1681.2002.03737.x. Clin Exp Pharmacol Physiol. 2002. PMID: 12165037 Review. - Calcitonin-derived carrier peptides.
Neundorf I, Beck-Sickinger AG. Neundorf I, et al. Curr Pharm Des. 2005;11(28):3661-9. doi: 10.2174/138161205774580723. Curr Pharm Des. 2005. PMID: 16305502 Review.
Cited by
- Conformation and lipid binding of a C-terminal (198-243) peptide of human apolipoprotein A-I.
Zhu HL, Atkinson D. Zhu HL, et al. Biochemistry. 2007 Feb 13;46(6):1624-34. doi: 10.1021/bi061721z. Biochemistry. 2007. PMID: 17279626 Free PMC article. - Acetyl-[Asn30,Tyr32]-calcitonin fragment 8-32 forms channels in phospholipid planar lipid membranes.
Meleleo D, Gallucci E, Picciarelli V, Micelli S. Meleleo D, et al. Eur Biophys J. 2007 Sep;36(7):763-70. doi: 10.1007/s00249-007-0150-6. Epub 2007 Mar 29. Eur Biophys J. 2007. PMID: 17393160 - Transmembrane glycine zippers: physiological and pathological roles in membrane proteins.
Kim S, Jeon TJ, Oberai A, Yang D, Schmidt JJ, Bowie JU. Kim S, et al. Proc Natl Acad Sci U S A. 2005 Oct 4;102(40):14278-83. doi: 10.1073/pnas.0501234102. Epub 2005 Sep 22. Proc Natl Acad Sci U S A. 2005. PMID: 16179394 Free PMC article. - Biophysical characterization of a recombinant aminopeptidase II from the thermophilic bacterium Bacillus stearothermophilus.
Wang TF, Lin MG, Lo HF, Chi MC, Lin LL. Wang TF, et al. J Biol Phys. 2014 Jan;40(1):25-40. doi: 10.1007/s10867-013-9332-x. Epub 2013 Oct 29. J Biol Phys. 2014. PMID: 24165863 Free PMC article.
References
- Altland, K., and P. Winter. 2003. Polyacrylamide gel electrophoresis followed by sodium dodecyl sulfate gradient polyacrylamide gel electrophoresis for the study of the dimmer to monomer transition of human transthyretin. Electrophoresis. 24:2265–2271. - PubMed
- Amodeo, P., A. Motta, G. Strazzullo, and M. A. Castiglione Morelli. 1999. Conformational flexibility in calcitonin: The dynamic properties of human and salmon calcitonin in solution. Journal Biomol. NMR. 13:161–174. - PubMed
- Arvinte, T., A. Cudd, and A. F. Drake. 1993. The structure and mechanism of formation of human calcitonin fibrils. J. Biol. Chem. 268:6415–6422. - PubMed
- Auluck, P. K., H. Y. Chan, J. Q. Trojanowski, V. M. Lee, and N. M. Bonini. 2002. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 295:865–868. - PubMed
- Barrow, C. J., and M. G. Zagorski. 1991. Solution structures of beta peptide and its constituent fragments: relation to amyloid deposition. Science. 253:180–182. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources