Type I interferon production enhances susceptibility to Listeria monocytogenes infection - PubMed (original) (raw)
Comparative Study
. 2004 Aug 16;200(4):437-45.
doi: 10.1084/jem.20040712. Epub 2004 Aug 9.
Supriya K Saha, Sagar A Vaidya, Kevin W Bruhn, Gustavo A Miranda, Brian Zarnegar, Andrea K Perry, Bidong O Nguyen, Timothy F Lane, Tadatsugu Taniguchi, Jeff F Miller, Genhong Cheng
Affiliations
- PMID: 15302901
- PMCID: PMC2211937
- DOI: 10.1084/jem.20040712
Comparative Study
Type I interferon production enhances susceptibility to Listeria monocytogenes infection
Ryan M O'Connell et al. J Exp Med. 2004.
Abstract
Numerous bacterial products such as lipopolysaccharide potently induce type I interferons (IFNs); however, the contribution of this innate response to host defense against bacterial infection remains unclear. Although mice deficient in either IFN regulatory factor (IRF)3 or the type I IFN receptor (IFNAR)1 are highly susceptible to viral infection, we show that these mice exhibit a profound resistance to infection caused by the Gram-positive intracellular bacterium Listeria monocytogenes compared with wild-type controls. Furthermore, this enhanced bacterial clearance is accompanied by a block in L. monocytogenes-induced splenic apoptosis in IRF3- and IFNAR1-deficient mice. Thus, our results highlight the disparate roles of type I IFNs during bacterial versus viral infections and stress the importance of proper IFN modulation in host defense.
Figures
Figure 1.
Induction of IFN-β by L. monocytogenes requires IRF3. (A) BMMs from C57BL/6 wild-type mice were infected with L. monocytogenes at a multiplicity of infection of 1 and IRF3 and p65 nuclear translocation were assayed at the indicated times by immunoblotting using nuclear extract. USF-2 was also assayed as a loading control. (B) MyD88 − / −, IRF3 − / −, IFNAR1 −/−, and wild-type BMMs were infected with L. monocytogenes as described above and assayed at the indicated time points for _IFN-_β gene expression by Q-PCR and (C) phospho-STAT1α/β, or total STAT1α/β by immunoblotting. Results shown are representative of at least two independent experiments, and Q-PCR results are expressed in relative expression units and have been normalized to L32 mRNA levels.
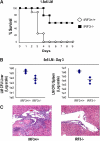
Figure 2.
Mice deficient in IRF3 have an enhanced ability to clear infection by L. monocytogenes. (A) IRF3 −/− (n = 8) and wild-type control mice (n = 8) were infected intravenously with 1.5 × 106 L. monocytogenes and viability was assayed daily for 9 d. (B) IRF3 −/− (n = 4) and wild-type mice (n = 4) were infected intravenously with 5 × 105 L. monocytogenes for 3 d, at which time the L. monocytogenes titer in the spleen and liver was assayed and presented as CFUs. (C) A portion of the IRF3 −/− and wild-type livers from B were fixed, sectioned, and subjected to a histological analysis after hematoxylin and eosin staining. All data are representative of at least two independent experiments.
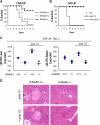
Figure 3.
Type I IFNs sensitize mice to L. monocytogenes infection. (A) IFNAR1 −/− (n = 13), IFNAR1 +/− (n = 8), and IFNAR1 +/+ (n = 14) mice were infected intravenously with 1.5 × 106 L. monocytogenes and viability was assayed daily for 9 d. (B) Wild-type mice were challenged with 5 × 105 L. monocytogenes (n = 6), 200 μg poly I:C (n = 6), or the combination of the two (n = 6), and viability was assayed daily for 9 d. (C) IFNAR1 − / − and wild-type control mice were infected intravenously with 5 × 105 L. monocytogenes either in the absence or presence of 200 μg poly I:C. After 3 d, the mice were killed and the L. monocytogenes titer was determined in the liver (n = 4) and spleen (n = 4), and presented as CFUs. (D) A portion of the livers from C were fixed, sectioned, and subjected to a histological analysis after hematoxylin and eosin staining. All data are representative of at least two independent experiments.
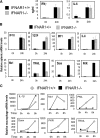
Figure 4.
Type I, but not Type II, IFN signaling is inhibited in _L. monocytogenes_–infected _IFNAR1−/−_mice. (A) The spleens and serum from IFNAR1 − / − and wild-type mice infected with 1.5 × 106 L. monocytogenes were removed 24 h after infection and serum IFN-γ and IL-6 levels were assayed by ELISA. (B) The splenocyte mRNA was subjected to a Q-PCR analysis to assay IP10, IRF1, TGTP, IL-6, Mx1, TRAIL, PKR, and Daxx expression. (C) IFNAR1 −/− and wild-type BMMs were infected with L. monocytogenes at a multiplicity of infection of 1 and assayed at the indicated time points for _IL-1_β, Mx1, PKR, Daxx, TRAIL, and PML gene expression by Q-PCR. Results shown are representative of at least two independent experiments. Q-PCR data are presented in relative expression units and have been normalized to L32 mRNA levels.
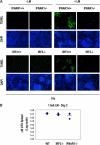
Figure 5.
_L. monocytogenes_–induced splenic apoptosis is dependent on type I IFNs. (A) Spleens from IFNAR1 − / −, IRF3 −/−, and wild-type mice that had been infected with 1.5 × 106 L. monocytogenes 48 h previously were removed and subjected to a TUNEL assay followed by DAPI staining of the nuclei. Pictures were taken using a 10× lens. (B) Portions of the spleens from wild-type (n = 3), IRF3 − / − (n = 3), and IFNAR1 − / − (n = 4) mice used for TUNEL assays in A were assayed and presented as CFUs 2 d after infection with 1.5 × 106 L. monocytogenes. All data represent three independent experiments.
Similar articles
- Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes.
Auerbuch V, Brockstedt DG, Meyer-Morse N, O'Riordan M, Portnoy DA. Auerbuch V, et al. J Exp Med. 2004 Aug 16;200(4):527-33. doi: 10.1084/jem.20040976. Epub 2004 Aug 9. J Exp Med. 2004. PMID: 15302899 Free PMC article. - Type I IFN induction in response to Listeria monocytogenes in human macrophages: evidence for a differential activation of IFN regulatory factor 3 (IRF3).
Reimer T, Schweizer M, Jungi TW. Reimer T, et al. J Immunol. 2007 Jul 15;179(2):1166-77. doi: 10.4049/jimmunol.179.2.1166. J Immunol. 2007. PMID: 17617610 - Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection.
Carrero JA, Calderon B, Unanue ER. Carrero JA, et al. J Exp Med. 2004 Aug 16;200(4):535-40. doi: 10.1084/jem.20040769. Epub 2004 Aug 9. J Exp Med. 2004. PMID: 15302900 Free PMC article. - Novel functions of type I interferons revealed by infection studies with Listeria monocytogenes.
Stockinger S, Decker T. Stockinger S, et al. Immunobiology. 2008;213(9-10):889-97. doi: 10.1016/j.imbio.2008.07.020. Epub 2008 Sep 2. Immunobiology. 2008. PMID: 18926303 Review. - Confounding roles for type I interferons during bacterial and viral pathogenesis.
Carrero JA. Carrero JA. Int Immunol. 2013 Dec;25(12):663-9. doi: 10.1093/intimm/dxt050. Epub 2013 Oct 24. Int Immunol. 2013. PMID: 24158954 Free PMC article. Review.
Cited by
- Type I IFN-mediated regulation of IL-1 production in inflammatory disorders.
Ludigs K, Parfenov V, Du Pasquier RA, Guarda G. Ludigs K, et al. Cell Mol Life Sci. 2012 Oct;69(20):3395-418. doi: 10.1007/s00018-012-0989-2. Epub 2012 Apr 24. Cell Mol Life Sci. 2012. PMID: 22527721 Free PMC article. Review. - Disease-promoting effects of type I interferons in viral, bacterial, and coinfections.
Davidson S, Maini MK, Wack A. Davidson S, et al. J Interferon Cytokine Res. 2015 Apr;35(4):252-64. doi: 10.1089/jir.2014.0227. Epub 2015 Feb 25. J Interferon Cytokine Res. 2015. PMID: 25714109 Free PMC article. Review. - Impact of lymphocyte apoptosis on the innate immune stages of infection.
Carrero JA, Unanue ER. Carrero JA, et al. Immunol Res. 2007;38(1-3):333-41. doi: 10.1007/s12026-007-0017-z. Immunol Res. 2007. PMID: 17917040 - Th1 cytokines are essential for placental immunity to Listeria monocytogenes.
Barber EM, Fazzari M, Pollard JW. Barber EM, et al. Infect Immun. 2005 Oct;73(10):6322-31. doi: 10.1128/IAI.73.10.6322-6331.2005. Infect Immun. 2005. PMID: 16177303 Free PMC article. - Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population.
Antonelli LR, Gigliotti Rothfuchs A, Gonçalves R, Roffê E, Cheever AW, Bafica A, Salazar AM, Feng CG, Sher A. Antonelli LR, et al. J Clin Invest. 2010 May;120(5):1674-82. doi: 10.1172/JCI40817. Epub 2010 Apr 12. J Clin Invest. 2010. PMID: 20389020 Free PMC article.
References
- Isaacs, A., and J. Lindenmann. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. B. Biol. Sci. 147:258–267. - PubMed
- Taniguchi, T., and A. Takaoka. 2002. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr. Opin. Immunol. 14:111–116. - PubMed
- Le Bon, A., and D.F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432–436. - PubMed
- Biron, C.A. 2001. Interferons alpha and beta as immune regulators–a new look. Immunity. 14:661–664. - PubMed
- Taniguchi, T., K. Ogasawara, A. Takaoka, and N. Tanaka. 2001. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19:623–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM008042/GM/NIGMS NIH HHS/United States
- R37 AI047868/AI/NIAID NIH HHS/United States
- R01 CA087924/CA/NCI NIH HHS/United States
- GM 08042/GM/NIGMS NIH HHS/United States
- R37 AI47868/AI/NIAID NIH HHS/United States
- T32 GM007185/GM/NIGMS NIH HHS/United States
- T32 GM007104/GM/NIGMS NIH HHS/United States
- S10 RR15862-01/RR/NCRR NIH HHS/United States
- R01 AI056154/AI/NIAID NIH HHS/United States
- R01 CA87924/CA/NCI NIH HHS/United States
- GM 07104/GM/NIGMS NIH HHS/United States
- GM 07185/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases