Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2) - PubMed (original) (raw)
Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2)
Victoria Valinluck et al. Nucleic Acids Res. 2004.
Abstract
Cytosine methylation in CpG dinucleotides is believed to be important in gene regulation, and is generally associated with reduced levels of transcription. Methylation-mediated gene silencing involves a series of DNA-protein and protein-protein interactions that begins with the binding of methyl-CpG binding proteins (MBPs) followed by the recruitment of histone-modifying enzymes that together promote chromatin condensation and inactivation. It is widely known that alterations in methylation patterns, and associated gene activities, are often found in human tumors. However, the mechanisms by which methylation patterns are altered are not currently understood. In this paper, we investigate the impact of oxidative damage to a methyl-CpG site on MBP binding by the selective placement of 8-oxoguanine (8-oxoG) and 5-hydroxymethylcytosine (HmC) in a MBP recognition sequence. Duplexes containing these specific modifications were assayed for binding to the methyl-CpG binding domain (MBD) of one member of the MBP family, methyl-CpG binding protein 2 (MeCP2). Our results reveal that oxidation of either a single guanine to 8-oxoG or of a single 5mC to HmC, significantly inhibits binding of the MBD to the oligonucleotide duplex, reducing the binding affinity by at least an order of magnitude. Oxidative damage to DNA could therefore result in heritable, epigenetic changes in chromatin organization.
Figures
Figure 1
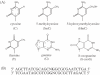
(A) Structures of cytosine, 5-methylcytosine, 5-hydroxymethylcytosine, thymine, guanine and 8-oxoG. (B) Sequence of ODN duplex used in EMSA. Replacement of position N with cytosine, 5mC or HmC in both strands yields duplexes C/C, 5mC/5mC and HmC/HmC, respectively. The duplexes are named based upon the modification at the N position, with the slash denoting that the modification is on different strands within the central CpG. Modification in the upper strand is indicated before the slash, whereas modification to the lower strand is indicated after the slash. Four duplexes containing 8-oxoG were used and are named in a manner similar to, e.g. the 5mC/5mC8oxoG duplex contains 5mC at position N in both the upper and lower strands with 8-oxoG replacing the boldface G in the lower strand. The duplex containing 5mC at position N in the upper strand and T at position N in the lower strand was given the name 5mC/T.
Figure 2
Binding of C/C, C/5mC, 5mC/5mC and 5mC/T duplexes to varying concentrations of MBD from 0 to 256 nM assayed via EMSA.
Figure 3
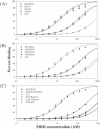
Non-linear regression of the plot of average percentage binding (determined from EMSA with three or more sets of titrations per duplex) and concentration of MBD for duplexes containing (A) normal purines within the CpG site, (B) HmC within the CpG site and (C) 8-oxoG within the CpG site.
Figure 4
Binding of 5mC/5mC, 5mC/HmC and 5mC/5mC8-oxoG duplexes to varying concentrations of MBD from 0 to 256 nM assayed via EMSA.
Figure 5
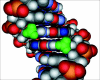
Molecular model of the sequence 5′-GCmCGGC-3′. The methyl groups of 5mC are depicted in green, and the N7 position of guanines within the methyl-CpG dinucleotide are depicted in dark blue. Multiple sites within the methylated CpG dinucleotide are needed for strong binding by the MBD. All four major sites of contact, two methyl groups and two hydrogen bond accepting nitrogens, are in the major groove of the DNA within close proximity of one another. Disruption of MBD binding results from oxidative damage to any one of these four sites.
Similar articles
- 5-halogenated pyrimidine lesions within a CpG sequence context mimic 5-methylcytosine by enhancing the binding of the methyl-CpG-binding domain of methyl-CpG-binding protein 2 (MeCP2).
Valinluck V, Liu P, Kang JI Jr, Burdzy A, Sowers LC. Valinluck V, et al. Nucleic Acids Res. 2005 May 25;33(9):3057-64. doi: 10.1093/nar/gki612. Print 2005. Nucleic Acids Res. 2005. PMID: 15917437 Free PMC article. - The solution structure of the domain from MeCP2 that binds to methylated DNA.
Wakefield RI, Smith BO, Nan X, Free A, Soteriou A, Uhrin D, Bird AP, Barlow PN. Wakefield RI, et al. J Mol Biol. 1999 Sep 3;291(5):1055-65. doi: 10.1006/jmbi.1999.3023. J Mol Biol. 1999. PMID: 10518942 - Altered chromatin structure associated with methylation-induced gene silencing in cancer cells: correlation of accessibility, methylation, MeCP2 binding and acetylation.
Nguyen CT, Gonzales FA, Jones PA. Nguyen CT, et al. Nucleic Acids Res. 2001 Nov 15;29(22):4598-606. doi: 10.1093/nar/29.22.4598. Nucleic Acids Res. 2001. PMID: 11713309 Free PMC article. - Methyl-CpG binding proteins in the nervous system.
Fan G, Hutnick L. Fan G, et al. Cell Res. 2005 Apr;15(4):255-61. doi: 10.1038/sj.cr.7290294. Cell Res. 2005. PMID: 15857580 Review. - Methyl-CpG-binding proteins. Targeting specific gene repression.
Ballestar E, Wolffe AP. Ballestar E, et al. Eur J Biochem. 2001 Jan;268(1):1-6. doi: 10.1046/j.1432-1327.2001.01869.x. Eur J Biochem. 2001. PMID: 11121095 Review.
Cited by
- Fine particulate matter‑induced cardiac developmental toxicity (Review).
Meng X, Du W, Sun Z. Meng X, et al. Exp Ther Med. 2024 Oct 29;29(1):6. doi: 10.3892/etm.2024.12756. eCollection 2025 Jan. Exp Ther Med. 2024. PMID: 39534282 Free PMC article. Review. - Network Analysis of Dysregulated Immune Response to COVID-19 mRNA Vaccination in Hemodialysis Patients.
Chang YS, Lee JM, Huang K, Vagts CL, Ascoli C, Edafetanure-Ibeh R, Huang Y, Cherian RA, Sarup N, Warpecha SR, Hwang S, Goel R, Turturice BA, Schott C, Martinez MH, Finn PW, Perkins DL. Chang YS, et al. Vaccines (Basel). 2024 Oct 7;12(10):1146. doi: 10.3390/vaccines12101146. Vaccines (Basel). 2024. PMID: 39460313 Free PMC article. - Cell-Free DNA Hydroxymethylation in Cancer: Current and Emerging Detection Methods and Clinical Applications.
Li JJN, Liu G, Lok BH. Li JJN, et al. Genes (Basel). 2024 Sep 3;15(9):1160. doi: 10.3390/genes15091160. Genes (Basel). 2024. PMID: 39336751 Free PMC article. Review. - Transcriptome-Wide 5-Methylcytosine Profiling of lncRNAs in the Mouse Cerebral Ischemia Model.
Zhang C, Gao J, Xiong D, Zhao Y. Zhang C, et al. Pharmaceuticals (Basel). 2024 Mar 18;17(3):384. doi: 10.3390/ph17030384. Pharmaceuticals (Basel). 2024. PMID: 38543171 Free PMC article. - Epigenetic landscape of 5-hydroxymethylcytosine and associations with gene expression in placenta.
Mortillo M, Kennedy EG, Hermetz KM, Burt AA, Marsit CJ. Mortillo M, et al. Epigenetics. 2024 Dec;19(1):2326869. doi: 10.1080/15592294.2024.2326869. Epub 2024 Mar 20. Epigenetics. 2024. PMID: 38507502 Free PMC article.
References
- Ehrlich M. and Wang,F.Y.-H. (1981) 5-Methylcytosine in eukaryotic DNA. Science, 212, 1350–1357. - PubMed
- Riggs A.D. and Jones,P.A. (1983) 5-Methylcytosine, gene regulation and cancer. Adv. Cancer Res., 40, 1–30. - PubMed
- Doerfler W. (1983) DNA methylation and gene activity. Ann. Rev. Biochem., 52, 93–124. - PubMed
- Antequera F., Boyes,J. and Bird,A. (1990) High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell, 62, 503–514. - PubMed
- Jaenisch R. and Bird,A. (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genet., 33, 245–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous