Measles virus interacts with and alters signal transduction in T-cell lipid rafts - PubMed (original) (raw)
Measles virus interacts with and alters signal transduction in T-cell lipid rafts
Elita Avota et al. J Virol. 2004 Sep.
Abstract
By a contact-dependent surface interaction, the measles virus (MV) glycoprotein complex induces a pronounced inhibition of T-cell proliferation. We now show that MV directly interacts with glycosphingolipid-enriched membrane microdomains on human primary T cells and alters recruitment and segregation of membrane proximal signaling components. Contact-dependent interference with T-cell receptor-stimulated tyrosine phosphorylation and Ca mobilization is a late event seen 24 h after MV treatment. In contrast, stimulated recruitment of pleckstrin homology domain-containing proteins such as Akt and Vav is inhibited early after MV contact, as is segregation of the activated Akt kinase from rafts. Tyrosine phosphorylation of the regulatory subunit of the phosphatidylinositol 3-kinase (PI3K), p85, is apparently normal then, yet this protein fails to partition to the lipid raft fraction, and this is associated with stable expression of its negative regulator Cbl-b. Thus, by interaction with lipid rafts, MV contact initially targets recruitment of PI3K by preventing stimulated Cbl-b degradation and activation of PI3K-dependent signaling components.
Figures
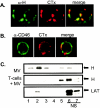
FIG. 1.
MV binds to lipid rafts on the T-cell membrane. (A) Primary T cells were cocultivated with MV strain WTF (at 4°C for 2 h), followed by GM1 staining with CTx-Alexa 594 conjugate and patching with anti-CTx serum at 37°C. After fixation, cells were stained with an MV-H-specific antibody (α-H) and an Alexa 488-conjugated secondary antibody. (B) Lipid raft staining and patching was done as described for panel A, and CD46 was detected by using a specific monoclonal antibody and Alexa 488-conjugated secondary antibody. (C) Extracts prepared from WTF alone (upper panel) or T cells cocultured with WTF (2 h, 4°C) were subjected to sucrose gradient centrifugation and analyzed for the partitioning of LAT (as a raft marker) (lower panel) or for the MV H protein (upper and middle panels) by Western blot analysis. NS, nonsoluble fraction at the top of the sucrose gradient.
FIG. 2.
MV affects stimulation-induced tyrosine phosphorylation in T cells at 24 h but not 2 h after exposure. (A) Extracts of primary T cells cocultivated with UV-inactivated MV WTF (or equivalent amounts of mock preparations) for 2 h at 4°C (left panels) or for 24 h at 37°C (right panels), followed by CD3/CD28 stimulation for 30 min, were analyzed for partitioning of LAT (NS, nonsoluble top fractions 6 and 7) (upper panels) or tyrosine-phosphorylated proteins (lower panels). (B) T cells treated with UV-inactivated WTF (or the corresponding amounts of the mock preparation) for 2 h at 4°C (left four panels) or for 24 h at 37°C (middle four panels) were cocultured with SEB-pulsed DCs for 20 min at 37°C, fixed, and stained with a phosphotyrosine-specific antibody and subsequently with an Alexa 488-conjugated secondary antibody. To visualize phosphotyrosine in T cells exposed to MV for 24 h, fluorescence was intensified. For quantification, the percentage of T cells translocating phosphotyrosine to the cortical membrane at the DC/T-cell interface was determined (right panel). Error bars indicate standard deviations. (C) The CD3-stimulated Ca2+ flux in T cells exposed to UV-inactivated WTF (red lines) for 2 h at 4°C (left panel) or for 24 h at 37°C (right panel) was determined. For all panels, the results of one representative experiment out of three independent experiments are shown.
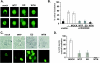
FIG. 3.
UV-WTF interaction prevents stimulated membrane accumulation of the Akt-PH domain. (A and C) Primary T cells nucleofected with a GFP-Akt-PH domain construct were pretreated with mock extracts, UV-inactivated WTF or ED (2 h, 4°C), or wortmannin (20 min) prior to CD3/CD28 stimulation (20 min) (A) or incubation with SEB-pulsed DCs (20 min, 37°C) (C) (two examples for each are shown). (B) The percentage of T cells translocating the GFP-Akt-PH domain to the cortical membrane after treatment with medium (unstim.) or with CD3/CD28 alone, with mock treatment, or in the presence of UV-inactivated WTF, UV-inactivated ED, or wortmannin (WTN) was determined. (D) Percentage of mock-, UV-inactivated WTF-, UV-inactivated ED-, or wortmannin-treated T cells translocating the GFP-Akt-PH domain to the cortical membrane at the DC/T-cell contact zone. For panels B and D, standard deviations were calculated from five different fluorescence images, each containing 20 to 50 GFP-positive cells.
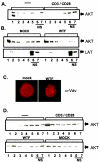
FIG. 4.
MV interferes with CD3/CD28-stimulated membrane recruitment of Akt and Vav proteins. (A) Lysates prepared from primary unstimulated (left lanes 1 to 7) or CD3/CD28-stimulated (right lanes 1 to 7) T cells were used to detect Akt protein. NS, nonsoluble. (B) Primary T cells were mock or MV (WTF strain) treated for 2 h at 4°C and subsequently CD3/CD28 stimulated for 30 min. Cell lysis, raft isolations, and anti-Akt and anti-LAT (raft marker) immunoblottings were performed. (C) T cells mock pretreated or pretreated with WTF for 2 h at 4°C were CD3/CD28 activated and stained for Vav protein. (D) Jurkat T cells were serum starved and left unstimulated (upper panel, left lanes 1 to 7) or stimulated with anti-CD3/CD28 for 30 min alone (upper panel, right lanes 1 to 7) or after pretreatment with mock extract or WTF (lower panel) for 2 h at 4°C. Lysate fractionation and anti-Akt immunoblotting were performed as described for panel A.
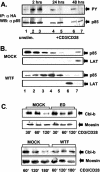
FIG. 5.
Tyrosine phosphorylation and raft recruitment of PI3K regulatory subunit p85 and Cbl-b expression after MV contact. (A) pCG-p85αHA-nucleofected primary T cells were left unstimulated (lane 1) or mock (lanes 2, 4, and 6) or WTF (lanes 3, 5, and 7) treated, followed by CD3/CD28 stimulation for 30 min (lanes 2 to 7). Lysates were immunoprecipitated (IP) with an anti-HA-antibody, followed by antiphosphotyrosine immunoblotting (WB) (upper panel) and anti-p85 reblotting (lower panel). (B) Lysates from CD3/CD28-stimulated primary T cells pretreated with mock extract (top panel) or WTF (2 h at 4°C) (third panel) were fractionated and analyzed for p85 partitioning by immunoblotting. LAT protein was used as a raft marker (second and fourth panels). (C) Extracts were prepared from primary T cells mock treated or treated with ED or WTF (upper panels) for 2 h at 4°C prior to CD3/CD28 stimulation. Cbl-b expression was analyzed after the time intervals indicated by Western blotting (upper panels); a moesin-specific antibody served as a loading control (bottom panels).
Similar articles
- CD38 signaling in T cells is initiated within a subset of membrane rafts containing Lck and the CD3-zeta subunit of the T cell antigen receptor.
Muñoz P, Navarro MD, Pavón EJ, Salmerón J, Malavasi F, Sancho J, Zubiaur M. Muñoz P, et al. J Biol Chem. 2003 Dec 12;278(50):50791-802. doi: 10.1074/jbc.M308034200. Epub 2003 Sep 30. J Biol Chem. 2003. PMID: 14523017 - Implication of phosphatidylinositol 3-kinase membrane recruitment in hydrogen peroxide-induced activation of PI3K and Akt.
Qin S, Chock PB. Qin S, et al. Biochemistry. 2003 Mar 18;42(10):2995-3003. doi: 10.1021/bi0205911. Biochemistry. 2003. PMID: 12627965 - Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells.
Fang D, Liu YC. Fang D, et al. Nat Immunol. 2001 Sep;2(9):870-5. doi: 10.1038/ni0901-870. Nat Immunol. 2001. PMID: 11526404 - CD16-mediated activation of phosphatidylinositol-3 kinase (PI-3K) in human NK cells involves tyrosine phosphorylation of Cbl and its association with Grb2, Shc, pp36 and p85 PI-3K subunit.
Cerboni C, Gismondi A, Palmieri G, Piccoli M, Frati L, Santoni A. Cerboni C, et al. Eur J Immunol. 1998 Mar;28(3):1005-15. doi: 10.1002/(SICI)1521-4141(199803)28:03<1005::AID-IMMU1005>3.0.CO;2-O. Eur J Immunol. 1998. PMID: 9541596
Cited by
- Activation of the N-Ras-PI3K-Akt-mTOR pathway by hepatitis C virus: control of cell survival and viral replication.
Mannová P, Beretta L. Mannová P, et al. J Virol. 2005 Jul;79(14):8742-9. doi: 10.1128/JVI.79.14.8742-8749.2005. J Virol. 2005. PMID: 15994768 Free PMC article. - Viral modulation of T-cell receptor signaling.
Jerome KR. Jerome KR. J Virol. 2008 May;82(9):4194-204. doi: 10.1128/JVI.00059-08. Epub 2008 Feb 20. J Virol. 2008. PMID: 18287237 Free PMC article. Review. No abstract available. - Inhibition of T-cell receptor signal transduction and viral expression by the linker for activation of T cells-interacting p12(I) protein of human T-cell leukemia/lymphoma virus type 1.
Fukumoto R, Dundr M, Nicot C, Adams A, Valeri VW, Samelson LE, Franchini G. Fukumoto R, et al. J Virol. 2007 Sep;81(17):9088-99. doi: 10.1128/JVI.02703-06. Epub 2007 Jun 20. J Virol. 2007. PMID: 17582004 Free PMC article. - c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses.
Thien CB, Langdon WY. Thien CB, et al. Biochem J. 2005 Oct 15;391(Pt 2):153-66. doi: 10.1042/BJ20050892. Biochem J. 2005. PMID: 16212556 Free PMC article. Review. - Transcriptomic Profiling of Virus-Host Cell Interactions following Chicken Anaemia Virus (CAV) Infection in an In Vivo Model.
Giotis ES, Rothwell L, Scott A, Hu T, Talbot R, Todd D, Burt DW, Glass EJ, Kaiser P. Giotis ES, et al. PLoS One. 2015 Aug 5;10(8):e0134866. doi: 10.1371/journal.pone.0134866. eCollection 2015. PLoS One. 2015. PMID: 26244502 Free PMC article.
References
- Acuto, E., and D. A. Cantrell. 2000. T cell activation and the cytoskeleton. Annu. Rev. Immunol. 18:165-184. - PubMed
- Anderson, R. G., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts and other lipid domains. Science 296:1821-1825. - PubMed
- Astier, A., M. C. Trescol-Biemont, O. Azocar, B. Lamouille, and C. Rabourdin-Combe. 2000. CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J. Immunol. 164:6091-6095. - PubMed
- Avota, E., A. Avots, N. Niewiesk, L. P. Kane, U. Bommhardt, V. ter Meulen, and S. Schneider-Schaulies. 2001. Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nature Med. 7:725-731. - PubMed
- Campbell, S. M., S. M. Crowe, and J. Mak. 2001. Lipid rafts and HIV-1: from viral entry to assembly of progeny virus. J. Clin. Virol. 22:217-227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous