Dynein/dynactin regulate metaphase spindle length by targeting depolymerizing activities to spindle poles - PubMed (original) (raw)
Dynein/dynactin regulate metaphase spindle length by targeting depolymerizing activities to spindle poles
Jedidiah Gaetz et al. J Cell Biol. 2004.
Abstract
During cell division metaphase spindles maintain constant length, whereas spindle microtubules continuously flux polewards, requiring addition of tubulin subunits at microtubule plus-ends, polewards translocation of the microtubule lattice, and removal of tubulin subunits from microtubule minus-ends near spindle poles. How these processes are coordinated is unknown. Here, we show that dynein/dynactin, a multi-subunit microtubule minus-end-directed motor complex, and NuMA, a microtubule cross-linker, regulate spindle length. Fluorescent speckle microscopy reveals that dynactin or NuMA inhibition suppresses microtubule disassembly at spindle poles without affecting polewards microtubule sliding. The observed uncoupling of these two components of flux indicates that microtubule depolymerization is not required for the microtubule transport associated with polewards flux. Inhibition of Kif2a, a KinI kinesin known to depolymerize microtubules in vitro, results in increased spindle microtubule length. We find that dynein/dynactin contribute to the targeting of Kif2a to spindle poles, suggesting a model in which dynein/dynactin regulate spindle length and coordinate flux by maintaining microtubule depolymerizing activities at spindle poles.
Figures
Figure 1.
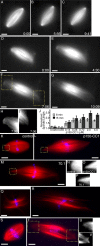
Dynein/dynactin inhibition increases the length of spindle microtubules in the presence or absence of centrosomes. (A–C) Tubulin distribution in untreated spindles during live recordings. (D–G) p150-CC1 addition (2 μM, ∼3 min before image at t = 0) caused spindles to increase in length. (H and I) Higher magnified spindle pole regions indicated in F (Videos 1 and 2). (J) p150-CC1 was added to assembled spindles, samples were fixed after 8 or 15 min, spindle lengths were measured (mean ± SD, n = 15, two independent experiments), and normalized to the length of untreated spindles (40 μm). (K–M) Spindles fixed 8 min after addition of control buffer (K), 2 μM p150-CC1 (L), or 1 mg/ml 70.1 (M) (tubulin, red; DNA, blue). (N–P) Higher magnified, contrast-adjusted regions indicated in K–M, respectively. (Q and R) Spindles assembled in 18 μm p50 dynamitin were treated with control buffer (Q) or 2 μm p150-CC1 (R) and fixed after 15 min (tubulin, red; DNA, blue). (S and T) Spindles assembled in the absence of centrosomes, around DNA-beads (tubulin, red; DNA, blue). (S) Buffer control. (T) p150-CC1–treated (2 μM, 8 min). (U and V) Higher magnified, contrast-adjusted regions indicated in R. Times are in min:s. Bars, 10 μm.
Figure 2.
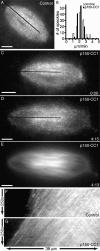
Dynactin inhibition with p150-CC1 suppresses microtubule depolymerization at spindle poles. Spindle microtubule dynamics were analyzed using fluorescent speckle microscopy. (A) Tubulin speckles in a control spindle. (B) Polewards velocities of tubulin speckles in control (white bars; 2.1 ± 0.3 μm/min, mean ± SD), or p150-CC1–treated (2 μM p150-CC1, black bars; 2.1± 0.2 μm/min, mean ± SD) spindles (n = 12 for each condition, 120 speckles). Velocities were binned in 0.5 μm/min increments. (C–E) Images from a time-lapse video of a p150-CC1–treated spindle (2 μM p150-CC1 added ∼3 min before image at t = 0) showing tubulin speckles (C and D) and tubulin distribution (E). The black lines in A, C, and D indicate the regions used to generate the kymographs shown in F and G, respectively (Videos 3 and 4). Times are in min:s. Bars, 10 μm.
Figure 3.
NuMA inhibition with LGN-N increases the length of spindle microtubules, in the presence or absence of centrosomes. (A–D) Tubulin distribution in an LGN-N–treated spindle (0.7 μM, added ∼3 min before image at t = 0) during live recordings (Video 5). (E) LGN-N was added to assembled spindles, samples were fixed after 8 or 15 min, spindle lengths were measured (mean ± SD, n = 15, two independent experiments), and normalized to the length of untreated spindles (40 μm). (F–H) Spindles assembled in the absence of centrosomes, around DNA beads (tubulin, red; DNA, blue). (F) Buffer control. (G) LGN-N–treated (2 μM, 8 min). (H) Higher magnified image of the region indicated in G. Times are in min:s. Bars, 10 μm.
Figure 4.
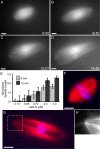
Kif2a is required for bipolar spindle assembly and the regulation of spindle microtubule length. (A) Western blot of Xenopus egg extracts stained with anti-Kif2a. Molecular weight standards are shown. (B–E) Anti-Kif2a inhibits bipolar spindle assembly. Anti-Kif2a (0.7 mg/ml; B and C) or control buffer (D and E) were added at the start of assembly reactions. (B and D) Tubulin alone. (C and E) Overlay (tubulin, red; DNA, blue). (F–K) Anti-Kif2a (0.7 mg/ml) was added to assembled spindles. (F and G) 8 min after antibody addition. Long microtubule bundles extended beyond (white arrowheads), and buckled (green arrowheads) within the spindle. (F) Tubulin alone. (G) Overlay (tubulin, red; DNA, blue). (H–K) Real-time analysis of a spindle treated with anti-Kif2a (added ∼3 min before image at t = 0; Video 6). Times are in min:s. Bars, 10 μm.
Figure 5.
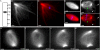
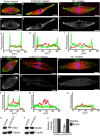
Treatment with p150-CC1 or 70.1 displaces Kif2a, but not MCAK from spindle poles. Assembled spindles, after addition of buffer, p150-CC1, or 70.1, were processed for immunofluorescence. MCAK staining in control (A and D), p150-CC1–treated (2 μM, 15 min; B and E), and 70.1-treated (1 mg/ml, 15 min; C and F) spindles. (A–C) Overlays (tubulin, red; DNA, blue; MCAK, green). (D–F) MCAK alone. (G–I) Line scans of fluorescence intensity (MCAK, green; tubulin, red; arbitrary units) across the pole to pole axis of the spindles shown in A–C. Kif2a staining in untreated (J and M), p150-CC1–treated (2 μM, 15 min; K and N), and 70.1-treated (1 mg/ml, 15 min; L and O) spindles. (J–L) Overlays (tubulin, red; DNA, blue; Kif2a, green). (M–O) Kif2a alone. (P–R) Line scans of fluorescence intensity (Kif2a, green; tubulin, red; arbitrary units) across the pole to pole axis of the spindles shown in (J–L). (S and T) Spindles were treated for 15 min with p150-CC1 (4 μM) or control buffer and the relative amount of Kif2a (S), or MCAK (T), and tubulin associated with partially purified spindle pellets was analyzed by immunoblotting. (U) Quantitation of spindle-associated Kif2a and MCAK relative to tubulin from measurements of immunoblot band intensities (mean ± SD, two independent experiments), normalized to intensities from untreated spindles. Bars, 10 μm.
Similar articles
- Poleward transport of Eg5 by dynein-dynactin in Xenopus laevis egg extract spindles.
Uteng M, Hentrich C, Miura K, Bieling P, Surrey T. Uteng M, et al. J Cell Biol. 2008 Aug 25;182(4):715-26. doi: 10.1083/jcb.200801125. Epub 2008 Aug 18. J Cell Biol. 2008. PMID: 18710923 Free PMC article. - Formation of spindle poles by dynein/dynactin-dependent transport of NuMA.
Merdes A, Heald R, Samejima K, Earnshaw WC, Cleveland DW. Merdes A, et al. J Cell Biol. 2000 May 15;149(4):851-62. doi: 10.1083/jcb.149.4.851. J Cell Biol. 2000. PMID: 10811826 Free PMC article. - Regional variation of microtubule flux reveals microtubule organization in the metaphase meiotic spindle.
Yang G, Cameron LA, Maddox PS, Salmon ED, Danuser G. Yang G, et al. J Cell Biol. 2008 Aug 25;182(4):631-9. doi: 10.1083/jcb.200801105. Epub 2008 Aug 18. J Cell Biol. 2008. PMID: 18710922 Free PMC article. - Role of NuMA in vertebrate cells: review of an intriguing multifunctional protein.
Sun QY, Schatten H. Sun QY, et al. Front Biosci. 2006 Jan 1;11:1137-46. doi: 10.2741/1868. Front Biosci. 2006. PMID: 16146802 Review. - [Dynein and dynactin as organizers of the system of cell microtubules].
Burakov AV, Nadezhdina ES. Burakov AV, et al. Ontogenez. 2006 Sep-Oct;37(5):323-39. Ontogenez. 2006. PMID: 17066975 Review. Russian.
Cited by
- The kinesin-13 proteins Kif2a, Kif2b, and Kif2c/MCAK have distinct roles during mitosis in human cells.
Manning AL, Ganem NJ, Bakhoum SF, Wagenbach M, Wordeman L, Compton DA. Manning AL, et al. Mol Biol Cell. 2007 Aug;18(8):2970-9. doi: 10.1091/mbc.e07-02-0110. Epub 2007 May 30. Mol Biol Cell. 2007. PMID: 17538014 Free PMC article. - Connecting neurodevelopment to neurodegeneration: a spotlight on the role of kinesin superfamily protein 2A (KIF2A).
Ruiz-Reig N, Hakanen J, Tissir F. Ruiz-Reig N, et al. Neural Regen Res. 2024 Feb;19(2):375-379. doi: 10.4103/1673-5374.375298. Neural Regen Res. 2024. PMID: 37488893 Free PMC article. Review. - Kinetochore-fiber lengths are maintained locally but coordinated globally by poles in the mammalian spindle.
Richter M, Neahring L, Tao J, Sutanto R, Cho NH, Dumont S. Richter M, et al. Elife. 2023 Jul 3;12:e85208. doi: 10.7554/eLife.85208. Elife. 2023. PMID: 37395732 Free PMC article. - Modeling reveals cortical dynein-dependent fluctuations in bipolar spindle length.
Mercadante DL, Manning AL, Olson SD. Mercadante DL, et al. Biophys J. 2021 Aug 3;120(15):3192-3210. doi: 10.1016/j.bpj.2021.05.030. Epub 2021 Jun 29. Biophys J. 2021. PMID: 34197801 Free PMC article. - Nuclear Mitotic Apparatus (NuMA) Interacts with and Regulates Astrin at the Mitotic Spindle.
Chu X, Chen X, Wan Q, Zheng Z, Du Q. Chu X, et al. J Biol Chem. 2016 Sep 16;291(38):20055-67. doi: 10.1074/jbc.M116.724831. Epub 2016 Jul 26. J Biol Chem. 2016. PMID: 27462074 Free PMC article.
References
- Cassimeris, L. 1999. Accessory protein regulation of microtubule dynamics throughout the cell cycle. Curr. Opin. Cell Biol. 11:134–141. - PubMed
- Desai, A., A. Murray, T.J. Mitchison, and C.E. Walczak. 1999. a. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61:385–412. - PubMed
- Desai, A., S. Verma, T.J. Mitchison, and C.E. Walczak. 1999. b. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 96:69–78. - PubMed
- Du, Q., P.T. Stukenberg, and I.G. Macara. 2001. A mammalian partner of inscuteable binds NuMA and regulates mitotic spindle organization. Nat. Cell Biol. 3:1069–1075. - PubMed
- Du, Q., L. Taylor, D.A. Compton, and I.G. Macara. 2002. LGN blocks the ability of NuMA to bind and stabilize microtubules. A mechanism for mitotic spindle assembly regulation. Curr. Biol. 12:1928–1933. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous