Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia - PubMed (original) (raw)
Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia
Shin-ichiro Kitajiri et al. J Cell Biol. 2004.
Abstract
Ezrin/radixin/moesin (ERM) proteins cross-link actin filaments to plasma membranes to integrate the function of cortical layers, especially microvilli. We found that in cochlear and vestibular sensory hair cells of adult wild-type mice, radixin was specifically enriched in stereocilia, specially developed giant microvilli, and that radixin-deficient (Rdx(-)(/)(-)) adult mice exhibited deafness but no obvious vestibular dysfunction. Before the age of hearing onset ( approximately 2 wk), in the cochlea and vestibule of Rdx(-)(/)(-) mice, stereocilia developed normally in which ezrin was concentrated. As these Rdx(-)(/)(-) mice grew, ezrin-based cochlear stereocilia progressively degenerated, causing deafness, whereas ezrin-based vestibular stereocilia were maintained normally in adult Rdx(-)(/)(-) mice. Thus, we concluded that radixin is indispensable for the hearing ability in mice through the maintenance of cochlear stereocilia, once developed. In Rdx(-)(/)(-) mice, ezrin appeared to compensate for radixin deficiency in terms of the development of cochlear stereocilia and the development/maintenance of vestibular stereocilia. These findings indicated the existence of complicate functional redundancy in situ among ERM proteins.
Figures
Figure 1.
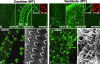
ERM proteins in the cochlea and vestibule in adult wild-type mice at the age of 5 wk. (a–c) Whole-mount immunofluorescence micrographs of the organ of Corti isolated from the cochlea with mAbs specific for ezrin, radixin, or moesin. The stereocilia of inner hair cells (IHC) and outer hair cells (OHC) are intensely stained with anti-radixin mAb (arrows). Ezrin and moesin are undetectable in the stereocilia, though moesin (moesin; green) is detected in large amounts in VE-cadherin–positive blood vessel endothelial cells (VE-cad; red) at a different focus plane (inset). Bar, 15 μm. (d and e) Comparison of the whole-mount radixin staining image with the scanning electron microscopic image of stereocilia on outer hair cells. Both radixin immunostaining (d) and scanning EM (e) reveal a highly organized array of stereocilia (arrows). Bars, 5 μm. (f–h) Whole-mount immunofluorescence micrographs of the crista ampullaris isolated from the vestibule with mAbs specific for ezrin, radixin, or moesin. Stereocilia are intensely stained with anti-radixin mAb, and weakly but reproducibly stained with anti-ezrin mAb (arrows). Moesin is detected only in the blood vessels at a different focus plane from radixin/ezrin-positive stereocilia (inset), as judged by the double-staining images for moesin (moesin; green) and VE-cadherin (VE-cad; red, inset). Bar, 15 μm. (i and j) Comparison of the whole-mount radixin staining image with the scanning electron microscopic image of stereocilia on vestibular hair cells. Radixin appears to concentrate along the entire length of long fragile stereocilia. Bars, 3 μm.
Figure 2.
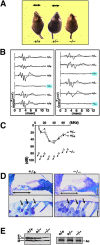
Deafness of Rdx −**/**− mice. (A) Loss of Preyer's reflex in Rdx −/− mice. Time-lapse photography captures Preyer's reflex in Rdx +/+ and Rdx +/− mice, head movements (arrows), but not in Rdx −/− mice. Two successive frames after a loud handclap (1-s interval) were superimposed. These 10-wk-old mice show similar growth rates. (B) ABR to stimuli of 70-dB SPL (20 kHz) in two sets of Rdx +/− intercross littermates (left, 5-wk-old; right 10-wk-old). Among 12 littermates in total, three show no ABR, and were afterwards genotyped as Rdx −/− mice (blue squares). (C) Hearing thresholds of 10-wk-old Rdx +/+, Rdx +/−, and Rdx −/− mice at various sound frequencies. Rdx +/+ and Rdx +/− mice show normal hearing thresholds (10–50 dB SPL), whereas Rdx −/− mice show profound deafness (hearing threshold, >70–90 dB SPL). (D) Toluidine blue–stained Epon semi-thin sections of the cochlea. No gross morphological difference is observed in the organ of Corti (double-headed arrows) including hair cells (single-headed arrows) between Rdx +/+ and Rdx −/− mice. Bars, 50 μm (top panels); 10 μm (bottom panels). (E) Western blot analysis of isolated cochleae of the Rdx +/+ , Rdx +/−, and Rdx −/− mice with anti-ERM pAb (TK89) that recognizes ezrin (E), radixin (R), and moesin (M) equally. In the Rdx −/− cochlea, radixin becomes undetectable without significant up-regulation of ezrin or moesin. Silver-stained bands of actin (Ac) in the same gels are present to show that an equal amount of cell lysate was applied in each lane.
Figure 3.
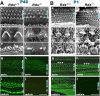
Stereocilia on Rdx +/+ and Rdx −**/**− cochlear hair cells of adult and newborn mice. (A) Scanning electron micrographs (a–f) and whole-mount immunofluorescence micrographs for radixin and ezrin (g–j) of the Rdx +/+ and Rdx −/− organ of Corti at 40 d of age (P40). The levels of outer hair cells (blue arrows) and inner hair cells (black arrows) are shown. At low magnification in the scanning electron micrograph of the Rdx +/+ organ of Corti (a), the luminal surface is characterized by three rows of outer hair cells (blue arrow) and one row of inner hair cells (black arrow). Outer hair cells bear stereocilia arranged regularly in the form of a letter W (c), and inner hair cells have more disorganized arrays of stereocilia (e). In the Rdx −/− organ of Corti (b), no significant abnormalities are detected in the cellular arrangements on the luminal surface, but the morphology of the stereocilia on both the outer (blue arrow) and inner (black arrow) hair cells is severely affected. In Rdx −/− outer hair cells, instead of regularly arranged stereocilia, 1–3 residual knoblike protrusions are observed on their apical surface (d). Rdx −/− inner hair cells bear several shorter fused irregularly shaped protrusions instead of long stereocilia (f). By immunostaining, in the Rdx −/− organ of Corti (h and j), no staining for radixin is detected without any increase in the staining intensities for ezrin. Bars, 10 μm (a and b); 2 μm (c and d); 1 μm (e and f); 20 μm (g–j). (B) Scanning electron micrographs (a–f) and whole-mount immunofluorescence micrographs for radixin and ezrin (g–j) of the Rdx +/+ and Rdx −/− organ of Corti at 1 d of age (P1). Scanning electron microscopic images of the cochlea of P1 Rdx −/− mice (b, d, and f) are indistinguishable from those in the Rdx +/+ mice (a, c, and e). In addition to stereocilia arrays carrying central tubulin-based kinocilium (arrows in c and d), hair cells as well as supporting cells are covered with large numbers of short conventional microvilli that have mostly disappeared in adult mice. Whole-mount immunostaining reveals that in the P1 Rdx +/+ organ of Corti, radixin is highly enriched in stereocilia of inner and outer hair cells as well as the apical surface of hair and supporting cells (i), but in contrast to adult mice ezrin is also detected in stereocilia, weakly but clearly (g, arrowheads). In the Rdx −/− organ of Corti, instead of radixin, ezrin is highly concentrated at stereocilia on both inner and outer hair cells (h, arrowheads). Bars, 10 μm (a and b); 2 μm (c and d); 1 μm (e and f); 20 μm (g–j).
Figure 4.
Postnatal degeneration of stereocilia on Rdx −**/**− cochlear hair cells. (A) Scanning electron micrographs (a–f) and whole-mount immunofluorescence micrographs for radixin and ezrin (g–j) of the organ of Corti isolated from Rdx +/+ and Rdx −/− mice at 6 d of age (P6). The levels of outer hair cells (blue arrows) and inner hair cells (black arrows) are shown. Scanning EM does not detect any significant differences between Rdx +/+ (a, c, and e) and Rdx −/− cochlea (b, d, and f). In contrast to P1 Rdx +/+ cochlea, in P6 Rdx +/+ cochlea, whole-mount immunostaining detects a weak signal of ezrin at radixin-enriched stereocilia on inner hair cells (black arrow), and a trace of ezrin in outer hair cells (blue arrow) (g and i). In Rdx −/− cochlea (h and j), ezrin is still highly concentrated at stereocilia on both inner hair cells (black arrow) and outer hair cells (blue arrow). Bars, 10 μm (a and b); 2 μm (c and d); 1 μm (e and f); 20 μm (g–j). (B) Scanning electron micrographs (a–f) and whole-mount immunofluorescence micrographs for radixin and ezrin (g–j) of the organ of Corti isolated from Rdx +/+ and Rdx −/− mice at 14 d of age (P14). Scanning EM detects the initial sign for the degeneration of stereocilia on outer hair cells (blue arrow) (a–d). The central part of the W-shaped row of stereocilia is lost, leaving discontinuous and disorganized arrays of stereocilia in Rdx −/− cochlea. At this stage, no significant defects can be observed in stereocilia on the inner hair cells (black arrow) (b and f). In P14 Rdx +/+ cochlea, ezrin is only weakly detected at radixin-enriched stereocilia on inner hair cells (black arrow), and is not detected in outer hair cells (blue arrow) (g and i). In Rdx −/− cochlea, ezrin was still concentrated in the degenerating stereocilia in large amounts in outer and inner hair cells (h). Bars, 10 μm (a and b); 2 μm (c and d); 1 μm (e and f); 20 μm (g–j).
Figure 5.
Ultrathin-section electron micrographs of stereocilia of cochlear outer hair cells of Rdx +/+ and Rdx −**/**− mice aged 3 wk. (A) Low power electron micrographs. All of the Rdx +/+ hair cells bear numerous stereocilia with normal appearance, whereas Rdx −/− hair cells were frequently characterized by abnormal 1–3 residual knoblike cellular protrusions on their apical surfaces. These images may correspond to the scanning electron micrographs of Fig. 3 A, c and d, respectively. Bars, 2 μm. (B) Rdx +/+ radixin-based stereocilia (a) and Rdx −/− ezrin-based stereocilia at various degeneration stages (b–d). Rdx −/− ezrin-based stereocilia contained highly-organized bundles of actin filaments as cores (b) that were indistinguishable in appearance from those in Rdx +/+ radixin-based stereocilia (a), and during the process of degeneration, these stereocilia as well as core bundles appeared to be fused to form abnormal thick and short protrusions (c and d). Bars, 0.5 μm.
Figure 6.
The vestibule and the balance function of 5-wk-old Rdx −**/**− mice. (A) Scanning EM of the Rdx +/+ and Rdx −/− crista ampullaris of the vestibule. The appearance of stereocilia (arrows) was indistinguishable between Rdx +/+ and Rdx −/− mice. Bar, 5 μm. (B) VOR. A mouse head was rotated sinusoidally and the eye position recorded by a CCD camera (left). The eye velocity was calculated from the change of eye position, and was fitted with sinusoidal curves (red lines). The gain was obtained by dividing the peak eye velocity by the peak head velocity. No difference is detected in the VOR between Rdx +/+ and Rdx −/− mice. The VOR gains of Rdx −/− mice are also normal at any frequency of the head rotation stimulus in Rdx +/+ and Rdx −/− mice (right). (C) Western blot analysis of isolated Rdx +/+ and Rdx −/− vestibule (mainly crista ampullaris) with anti-ERM pAb (TK89) that recognizes ezrin, radixin, and moesin with almost the same affinity. In Rdx −/− vestibule, radixin (R) became undetectable without significant up-regulation of ezrin (E) or moesin (M). Silver-stained bands of actin (Ac) are presented on the right to show that equal amounts of cell lysate were applied in each lane. (D) Whole-mount immunostaining of the crista ampullaris isolated from the Rdx +/+ and Rdx −/− vestibules with ezrin-, radixin- and moesin-specific antibodies. In the Rdx +/+ crista ampullaris, radixin is highly concentrated in the stereocilia where ezrin is detected weakly but reproducibly (inset, a frozen section stained with anti-ezrin antibody). Moesin is detected only in the blood vessels at a different focus plane from stereocilia (inset), as judged by the double staining images for moesin (moesin; green) and VE-cadherin (VE-cad; red) (inset). By contrast, in the Rdx −/− crista ampullaris, the concentration of ezrin in the stereocilia is significantly increased. All samples were treated under completely identical conditions, paying special attention to ensure that the signals were not saturated. Bar, 40 μm.
Similar articles
- CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI.
Salles FT, Andrade LR, Tanda S, Grati M, Plona KL, Gagnon LH, Johnson KR, Kachar B, Berryman MA. Salles FT, et al. Cytoskeleton (Hoboken). 2014 Jan;71(1):61-78. doi: 10.1002/cm.21159. Epub 2013 Dec 10. Cytoskeleton (Hoboken). 2014. PMID: 24285636 Free PMC article. - Mutations of the RDX gene cause nonsyndromic hearing loss at the DFNB24 locus.
Khan SY, Ahmed ZM, Shabbir MI, Kitajiri S, Kalsoom S, Tasneem S, Shayiq S, Ramesh A, Srisailpathy S, Khan SN, Smith RJ, Riazuddin S, Friedman TB, Riazuddin S. Khan SY, et al. Hum Mutat. 2007 May;28(5):417-23. doi: 10.1002/humu.20469. Hum Mutat. 2007. PMID: 17226784 - Mutations of the mouse ELMO domain containing 1 gene (Elmod1) link small GTPase signaling to actin cytoskeleton dynamics in hair cell stereocilia.
Johnson KR, Longo-Guess CM, Gagnon LH. Johnson KR, et al. PLoS One. 2012;7(4):e36074. doi: 10.1371/journal.pone.0036074. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558334 Free PMC article. - [ERM (ezrin/radixin/moesin) as crosslinkers between actin filaments and plasma membranes].
Tsukita S. Tsukita S. Tanpakushitsu Kakusan Koso. 1996 Sep;41(12 Suppl):1899-905. Tanpakushitsu Kakusan Koso. 1996. PMID: 8890653 Review. Japanese. No abstract available. - ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction.
Tsukita S, Yonemura S. Tsukita S, et al. Curr Opin Cell Biol. 1997 Feb;9(1):70-5. doi: 10.1016/s0955-0674(97)80154-8. Curr Opin Cell Biol. 1997. PMID: 9013673 Review.
Cited by
- Two Sides of the Coin: Ezrin/Radixin/Moesin and Merlin Control Membrane Structure and Contact Inhibition.
Michie KA, Bermeister A, Robertson NO, Goodchild SC, Curmi PMG. Michie KA, et al. Int J Mol Sci. 2019 Apr 23;20(8):1996. doi: 10.3390/ijms20081996. Int J Mol Sci. 2019. PMID: 31018575 Free PMC article. Review. - Emerging role for ERM proteins in cell adhesion and migration.
Arpin M, Chirivino D, Naba A, Zwaenepoel I. Arpin M, et al. Cell Adh Migr. 2011 Mar-Apr;5(2):199-206. doi: 10.4161/cam.5.2.15081. Epub 2011 Mar 1. Cell Adh Migr. 2011. PMID: 21343695 Free PMC article. Review. - Function and expression pattern of nonsyndromic deafness genes.
Hilgert N, Smith RJ, Van Camp G. Hilgert N, et al. Curr Mol Med. 2009 Jun;9(5):546-64. doi: 10.2174/156652409788488775. Curr Mol Med. 2009. PMID: 19601806 Free PMC article. Review. - Rab11a Is Essential for the Development and Integrity of the Stereocilia and Kinocilia in the Mammalian Organ of Corti.
Knapp L, Sun H, Wang YM, Chen BJ, Lin X, Gao N, Chen P, Ren D. Knapp L, et al. eNeuro. 2023 Jun 5;10(6):ENEURO.0420-22.2023. doi: 10.1523/ENEURO.0420-22.2023. Print 2023 Jun. eNeuro. 2023. PMID: 37225424 Free PMC article. - Keratan sulfate, an electrosensory neurosentient bioresponsive cell instructive glycosaminoglycan.
Melrose J. Melrose J. Glycobiology. 2024 Apr 1;34(3):cwae014. doi: 10.1093/glycob/cwae014. Glycobiology. 2024. PMID: 38376199 Free PMC article. Review.
References
- Alagramam, K.N., C.L. Murcia, H.Y. Kwon, K.S. Pawlowski, C.G. Wright, and R.P. Woychik. 2001. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat. Genet. 27:99–102. - PubMed
- Avraham, K.B., T. Hasson, K.P. Steel, D.M. Kingsley, L.B. Russell, M.S. Mooseker, N.G. Copeland, and N.A. Jenkins. 1995. The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat. Genet. 11:369–375. - PubMed
- Belyantseva, I.A., V. Labay, E.T. Boger, A.J. Griffith, and T.B. Friedman. 2003. Stereocilia: the long and the short of it. Trends Mol. Med. 9:458–461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous