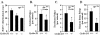Cyclin D1 genetic heterozygosity regulates colonic epithelial cell differentiation and tumor number in ApcMin mice - PubMed (original) (raw)
. 2004 Sep;24(17):7598-611.
doi: 10.1128/MCB.24.17.7598-7611.2004.
Chenguang Wang, Zhiping Li, Chris Albanese, Mahadev Rao, Dolores Di Vizio, Salimuddin Shah, Stephen W Byers, Radma Mahmood, Leonard H Augenlicht, Robert Russell, Richard G Pestell
Affiliations
- PMID: 15314168
- PMCID: PMC507010
- DOI: 10.1128/MCB.24.17.7598-7611.2004
Cyclin D1 genetic heterozygosity regulates colonic epithelial cell differentiation and tumor number in ApcMin mice
James Hulit et al. Mol Cell Biol. 2004 Sep.
Erratum in
- Mol Cell Biol. 2005 Jan;25(1):523
Abstract
Constitutive beta-catenin/Tcf activity, the primary transforming events in colorectal carcinoma, occurs through induction of the Wnt pathway or APC gene mutations that cause familial adenomatous polyposis. Mice carrying Apc mutations in their germ line (ApcMin) develop intestinal adenomas. Here, the crossing of ApcMin with cyclin D1-/- mice reduced the intestinal tumor number in animals genetically heterozygous or nullizygous for cyclin D1. Decreased tumor number in the duodenum, intestines, and colons of ApcMin/cyclin D1+/- mice correlated with reduced cellular proliferation and increased differentiation. Cyclin D1 deficiency reduced DNA synthesis and induced differentiation of colonic epithelial cells harboring mutant APC but not wild-type APC cells in vivo. In previous studies, the complete loss of cyclin D1 through homozygous genetic deletion conveyed breast tumor resistance. The protection of mice, genetically predisposed to intestinal tumorigenesis, through cyclin D1 heterozygosity suggests that modalities that reduce cyclin D1 abundance could provide chemoprotection.
Copyright 2004 American Society for Microbiology
Figures
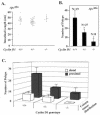
FIG. 1.
Cyclin D1 deficiency reduces colonic polyp formation. (A) Scatter plot showing the distribution of intestinal lengths as determined for the cyclin D1 wt, heterozygous, or nullizygous genotypes. (B) Polyp formation in ApcMin mice genetically deficient for cyclin D1. The mean number of colon polyps for each cyclin D1 genotype in the ApcMin genetic background is shown. Asterisks indicate P values <0.01. (C) Mean number of proximal and distal colon polyps for each cyclin D1 genotype. Error bars indicate standard errors of the means.
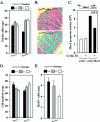
FIG. 2.
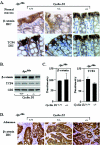
Intestinal cellular proliferation and differentiation in ApcMin mice genetically deficient for cyclin D1. (A) Goblet cells were stained with Alcian blue and counted. The number is shown as mean and standard error of the mean/crypt for each cyclin D1 genotype in the Apcwt and ApcMin genetic backgrounds (data comparisons are based on the following: ApcMin mice, total of 20 animals [8 cyclin D1+/+ mice, 80 crypts counted; 4 cyclin D1+/− mice, 22 crypts counted; 8 cyclin D1−/− mice, 75 crypts counted]; Apcwt mice, total of 11 animals [4 cyclin D1+/+ mice, 40 crypts; 3 cyclin D1+/− mice, 24 crypts; 4 cyclin D1−/− mice, 33 crypts]). (B) Analysis of colon epithelial goblet cells by Alcian blue staining in ApcMin mice by cyclin D1 genotype, with two cross-sectioned representative examples shown. (C) 293T cells were transfected with Muc2 promoter-driven luciferase reporter plasmid (pGL2-Muc2). Cyclin D1 cotransfection represses reporter activity in a Muc2 promoter-specific manner. pGL2 served as a reporter control. (D) Total colon epithelial cell counts per crypt in each genotype. (E) BrdU-positive cells per crypt.
FIG. 3.
Cyclin D1 heterozygosity reduces duodenal polyp number in ApcMin mice. (A) In vivo BrdU staining of the duodenum (n = 9 animals), shown as mean and standard error of the mean/crypt, in ApcMin mice by cyclin D1 genotype (13 weeks). (B to D) Mean numbers of polyps in ApcMin/cyclin D1wt and heterozygote mice (data are based on comparisons from nine mice [four cyclin D1+/+ and five _cyclin D1+/−_]) overall (B), in the duodenum (C), and in the jejunum-ileum (D). Asterisks indicate P values <0.05.
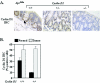
FIG. 4.
Cyclin D1 abundance in ApcMin/_cyclin D1_-deficient intestinal epithelium. (A) Cyclin D1 immunoreactivity in nonadenomatous colon mucosa for each cyclin D1 genotype (n = 24 animals; eight tissue preparations from each genotype). IHC, immunohistochemistry. (B) Bar graph comparing levels of cyclin D1 immunoreactivity in colon mucosa and polyps from cyclin D1 wt and heterozygote mice (n = 24; means and standard errors of the means are shown).
FIG. 5.
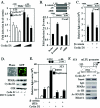
Genetic cyclin D1 deficiency increases PPARγ abundance in ApcMin intestinal epithelium. (A) Immunohistochemical (IHC) staining of duodenal epithelia of ApcMin mice. (B) Representative Western blot analysis of PPARγ1 in colonic epithelium in ApcMin mice (comparisons were made with 10 animals [4 cyclin D1+/+, 4 cyclin D1+/−, and 2 _cyclin D1−/−_] for each cyclin D1 genotype, two representative examples are shown for each genotype, and each lane represents lysate preparations made from individual animals with GDI as a loading control). (C) Analysis of mean PPARγ1 protein levels by densitometry (wt set to 1; mean and standard error of the mean; 13 weeks), normalized to GDI loading control. Asterisks indicate P values <0.05.
FIG. 6.
β-Catenin/Tcf expression in intestinal epithelium in ApcMin mice genetically deficient for cyclin D1. (A) Immunohistochemistry (IHC) of β-catenin and Tcf4 in normal colon from ApcMin mice for each cyclin D1 genotype (one representative example of each, from an assessment based on 24 animals and eight tissue preparations from each genotype, is shown). The image of normal mucosa for cyclin D1+/+ mice also contains adenomatous tissue. (B) Two representative examples are displayed, each prepared from individual animals. (C) Mean data from immunohistochemistry Western analysis for β-catenin and Tcf4 normalized to GDI loading control (wt set to 100) (n = 10). Error bars indicate standard errors of the means. (D) Immunohistochemistry of β-catenin in colon adenoma from ApcMin mice for each cyclin D1 genotype.
FIG. 7.
Cyclin D1 inhibits β-catenin-dependent signaling to the PPARγ1 promoter. (A) Cyclin D1 (150 or 300 ng) and β-catenin S33 (450 ng) were cotransfected with the PPARγ1 promoter. The luciferase reporter data are means and standard errors of the means. (B) CaCo2 colon cancer cells were cotransfected with the PPARγ1 promoter reporter and DNA encoding wt β-catenin and mutants. The luciferase reporter data are means and standard errors of the means. (C) CaCo2 cells were transfected with cyclin D1 and/or β-catenin together with the PPARγ1 promoter. The luciferase reporter data are presented as means and standard errors of the means from three independent experiments. (D) Cyclin D1 null mouse embryo fibroblasts were infected with cyclin D1 virus. Lysates were prepared and subjected to Western blot assay for PPARγ and cyclin D1. GDI served as a loading control. MSCV, murine stem cell virus. (E) The PPARγ-responsive reporter (AOX)3LUC was coexpressed with an expression vector for PPARγ1 (120 ng) and 150 or 300 ng of cyclin D1 in the presence of the PPARγ1 ligand (troglitazone) in SW480 colon cancer cells. The luciferase reporter data are means and standard errors of the means; all experiments were repeated six times. DMSO, dimethyl sulfoxide. (F) ChIP assays were performed on cyclin D1+/+ and cyclin D1−/− 3T3 cells, and immunoprecipitation was conducted with antibodies as indicated. The final DNA extractions were amplified by using pairs of primers to the PPRE region of the mouse LPL gene. IgG, immunoglobulin G.
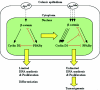
FIG. 8.
Cyclin D1 function in ApcMin CRC growth control. In cell with an Apcwt background, PPARγ provides negative feedback repression for β-catenin/Tcf4 signaling pathway-induced cell proliferation. Mutation of the Apc gene results in abnormal β-catenin translocation and overexpression of cyclin D1 in the nucleus. Overexpression of cyclin D1 further mediates _ApcMin_-dependent inhibition of colonic epithelial cell differentiation as assessed by goblet cell formation and _ApcMin_-dependent DNA synthesis.
Similar articles
- Colon epithelial cell differentiation is inhibited by constitutive c-myb expression or mutant APC plus activated RAS.
Ramsay RG, Ciznadija D, Sicurella C, Reyes N, Mitchelhill K, Darcy PK, D'Abaco G, Mantamadiotis T. Ramsay RG, et al. DNA Cell Biol. 2005 Jan;24(1):21-9. doi: 10.1089/dna.2005.24.21. DNA Cell Biol. 2005. PMID: 15684716 - Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells.
Tetsu O, McCormick F. Tetsu O, et al. Nature. 1999 Apr 1;398(6726):422-6. doi: 10.1038/18884. Nature. 1999. PMID: 10201372 - Down-regulation of beta-catenin TCF signaling is linked to colonic epithelial cell differentiation.
Mariadason JM, Bordonaro M, Aslam F, Shi L, Kuraguchi M, Velcich A, Augenlicht LH. Mariadason JM, et al. Cancer Res. 2001 Apr 15;61(8):3465-71. Cancer Res. 2001. PMID: 11309309 - Beta-catenin--a linchpin in colorectal carcinogenesis?
Wong NA, Pignatelli M. Wong NA, et al. Am J Pathol. 2002 Feb;160(2):389-401. doi: 10.1016/s0002-9440(10)64856-0. Am J Pathol. 2002. PMID: 11839557 Free PMC article. Review. - Cytoskeleton out of the cupboard: colon cancer and cytoskeletal changes induced by loss of APC.
Näthke I. Näthke I. Nat Rev Cancer. 2006 Dec;6(12):967-74. doi: 10.1038/nrc2010. Epub 2006 Nov 9. Nat Rev Cancer. 2006. PMID: 17093505 Review.
Cited by
- The canonical Wnt signalling pathway and its APC partner in colon cancer development.
Schneikert J, Behrens J. Schneikert J, et al. Gut. 2007 Mar;56(3):417-25. doi: 10.1136/gut.2006.093310. Epub 2006 Jul 13. Gut. 2007. PMID: 16840506 Free PMC article. Review. No abstract available. - Kinase-independent role of cyclin D1 in chromosomal instability and mammary tumorigenesis.
Casimiro MC, Di Sante G, Crosariol M, Loro E, Dampier W, Ertel A, Yu Z, Saria EA, Papanikolaou A, Li Z, Wang C, Addya S, Lisanti MP, Fortina P, Cardiff RD, Tozeren A, Knudsen ES, Arnold A, Pestell RG. Casimiro MC, et al. Oncotarget. 2015 Apr 20;6(11):8525-38. doi: 10.18632/oncotarget.3267. Oncotarget. 2015. PMID: 25940700 Free PMC article. - Cyclin D1 repression of nuclear respiratory factor 1 integrates nuclear DNA synthesis and mitochondrial function.
Wang C, Li Z, Lu Y, Du R, Katiyar S, Yang J, Fu M, Leader JE, Quong A, Novikoff PM, Pestell RG. Wang C, et al. Proc Natl Acad Sci U S A. 2006 Aug 1;103(31):11567-72. doi: 10.1073/pnas.0603363103. Epub 2006 Jul 24. Proc Natl Acad Sci U S A. 2006. PMID: 16864783 Free PMC article. - Thyroid hormone receptors regulate adipogenesis and carcinogenesis via crosstalk signaling with peroxisome proliferator-activated receptors.
Lu C, Cheng SY. Lu C, et al. J Mol Endocrinol. 2010 Mar;44(3):143-54. doi: 10.1677/JME-09-0107. Epub 2009 Sep 9. J Mol Endocrinol. 2010. PMID: 19741045 Free PMC article. Review.
References
- Albanese, C., M. D'Amico, A. T. Reutens, M. Fu, G. Watanabe, R. J. Lee, R. N. Kitsis, B. Henglein, M. Avantaggiati, K. Somasundaram, B. Thimmapaya, and R. G. Pestell. 1999. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J. Biol. Chem. 274:34186-34195. - PubMed
- Albanese, C., A. Reutens, M. D'Amico, B. Boumediene, M. Fu, T. Link, R. Nicholson, R. A. Depinho, and R. G. Pestell. 2000. Sustained mammary gland directed ponasterone A-inducible expression in transgenic mice. FASEB J. 14:877-884. - PubMed
- Arber, N., Y. Doki, E. K.-H. Han, A. Sgambato, P. Zhou, N.-H. Kim, T. Delohery, M. G. Klein, P. R. Holt, and I. B. Weinstein. 1997. Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res. 57:1569-1574. - PubMed
- Bartkova, J., J. Lukas, M. Strauss, and J. Bartek. 1994. The PRAD-1/cyclin D1 oncogene product accumulates in a subset of colorectal carcinoma. Int. J. Cancer 58:568-573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA075503/CA/NCI NIH HHS/United States
- R03AG20337/AG/NIA NIH HHS/United States
- R01CA75503/CA/NCI NIH HHS/United States
- P30 CA051008/CA/NCI NIH HHS/United States
- R01 CA070896/CA/NCI NIH HHS/United States
- P30 CA51008-13/CA/NCI NIH HHS/United States
- R01CA86071/CA/NCI NIH HHS/United States
- R01CA70896/CA/NCI NIH HHS/United States
- R01CA86072/CA/NCI NIH HHS/United States
- R01 CA086072/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous