The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response - PubMed (original) (raw)
The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response
Jan Karlseder et al. PLoS Biol. 2004 Aug.
Abstract
The telomeric protein TRF2 is required to prevent mammalian telomeres from activating DNA damage checkpoints. Here we show that overexpression of TRF2 affects the response of the ATM kinase to DNA damage. Overexpression of TRF2 abrogated the cell cycle arrest after ionizing radiation and diminished several other readouts of the DNA damage response, including phosphorylation of Nbs1, induction of p53, and upregulation of p53 targets. TRF2 inhibited autophosphorylation of ATM on S1981, an early step in the activation of this kinase. A region of ATM containing S1981 was found to directly interact with TRF2 in vitro, and ATM immunoprecipitates contained TRF2. We propose that TRF2 has the ability to inhibit ATM activation at telomeres. Because TRF2 is abundant at chromosome ends but not elsewhere in the nucleus, this mechanism of checkpoint control could specifically block a DNA damage response at telomeres without affecting the surveillance of chromosome internal damage.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
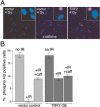
Figure 1. TRF2 Inhibits the IR-Induced Cell Cycle Arrest
(A) Retrovirally infected IMR90 cells were treated with 4 Gy IR (left and right) or treated with 4 Gy IR and exposed to caffeine (10 mM) directly after irradiation (middle). After 16 h, during which the cells were incubated in 1 μg/ml colcemide, the DNA was stained with DAPI and mitotic cells were identified by immunofluorescence with an antibody to phosphorylated histone H3. (B) Quantification of bypass of IR-induced cell cycle arrest. The mean percentage of phosphorylated histone H3-positive cells and SDs from three experiments are given. The low maximal incidence of phosphorylated H3-positive nuclei (approximately 18%) is due to loss of mitotic cells during processing; loss of mitotic cells occurred at the same level in control and experimental samples.
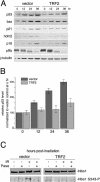
Figure 2. Effect of TRF2 on Downstream Readouts of the IR-Induced ATM Response
(A) Retrovirally infected IMR90 cells were exposed to 5 Gy IR and harvested after 0, 12, 24, and 36 h. Levels of p53, Bax, p21, Hdm2, p16, pRB, and γ-tubulin (loading control) were detected by immunoblotting of equal cell number equivalents. (B) Amount of p53 protein at the indicated timepoints (hours) was determined by densitometry of p53 immunoblots such as shown in (A). Amounts were normalized to the vector control at 0 h. Mean values from three experiments and standard deviations are shown. (C) Retrovirally infected IMR90 cells were exposed to 20 Gy IR and harvested after 45 min. Nbs1 was immunoprecipitated and subsequently detected by immunoblotting using a general Nbs1 antibody and a phosphospecific Nbs1 S343 antibody. IPs were treated with λ-phosphatase where indicated.
Figure 3. Effect of TRF2 on IR-Induced ATM Phosphorylation
(A) Overexpression of TRF2 inhibits IR-induced phosphorylation of transfected ATM in 293T cells. 293T cells co-transfected with ATM and either TRF2, TRF1, or vector were treated with the indicated doses of IR. After a 30-min recovery, cells were harvested and immunoblot analysis was performed on whole-cell lysates. (B) Overexpression of TRF2 inhibits IR-induced phosphorylation of endogenous ATM in primary fibroblasts. IMR90 primary fibroblasts infected with a retroviral construct expressing TRF2 or an empty virus were treated with the indicated doses of IR. After a 1 h recovery, cells were harvested and ATM was immunoprecipitated from whole-cell lysates. Immunoblot analysis was performed on immunoprecipitated ATM.
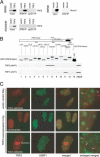
Figure 4. TRF2 Interacts with the ATM Kinase In Vivo and In Vitro and TRF2 Does Not Localize to IRIF
(A) Co-IP of TRF2 with ATM. Protein extracts from IMR90 cells and A-T cells (AG02496) infected with an empty virus or a TRF2-overexpressing virus were incubated with anti-ATM or anti-Cyclin D1 antibodies as indicated, and TRF2 was detected in the IP pellets by immunoblotting. The right panel represents IPs with anti-ATM antibodies from IMR90 cells infected with a retrovirus overexpressing Nova1 or the empty vector and detection of Nova1 by immunoblotting. For each extract 1% of the IP input (input) was processed for immunoblotting in parallel. (B) Bacterially expressed ATM–GST fusion proteins were purified on glutathione agarose beads and visualized by Western blotting with anti-GST antibody (Upstate Biotechnology [Lake Placid, New York, United States] #06–332) (top). Unfused GST was run on a separate gel because of its low molecular weight. Equal amounts of fusion proteins and GST alone were incubated with purified baculoviral TRF2 (middle) or TRF1 (bottom), bound to glutathione beads, spun down, washed, and bound proteins were processed for immunoblotting with an anti-TRF1 or anti-TRF2 serum. (C) TRF2 does not localize to IRIFS. IMR90 primary fibroblasts infected with a retroviral construct expressing TRF2 or an empty virus were treated with 5 Gy IR. After a 90 min recovery, cells were fixed and processed for immunofluorescence with or without Triton X-100 extraction before fixation. Arrowheads denote foci of TRF2 signal previously demonstrated to represent telomeres. When overexpressed, some TRF2 is localized to nucleolus.
Similar articles
- DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion.
Celli GB, de Lange T. Celli GB, et al. Nat Cell Biol. 2005 Jul;7(7):712-8. doi: 10.1038/ncb1275. Epub 2005 Jun 19. Nat Cell Biol. 2005. PMID: 15968270 - TRF2 dysfunction elicits DNA damage responses associated with senescence in proliferating neural cells and differentiation of neurons.
Zhang P, Furukawa K, Opresko PL, Xu X, Bohr VA, Mattson MP. Zhang P, et al. J Neurochem. 2006 Apr;97(2):567-81. doi: 10.1111/j.1471-4159.2006.03779.x. Epub 2006 Mar 15. J Neurochem. 2006. PMID: 16539655 - Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1.
Denchi EL, de Lange T. Denchi EL, et al. Nature. 2007 Aug 30;448(7157):1068-71. doi: 10.1038/nature06065. Epub 2007 Aug 8. Nature. 2007. PMID: 17687332 - DNA damage responses in neural cells: Focus on the telomere.
Zhang P, Dilley C, Mattson MP. Zhang P, et al. Neuroscience. 2007 Apr 14;145(4):1439-48. doi: 10.1016/j.neuroscience.2006.11.052. Epub 2007 Jan 4. Neuroscience. 2007. PMID: 17207936 Free PMC article. Review.
Cited by
- Processing by MRE11 is involved in the sensitivity of subtelomeric regions to DNA double-strand breaks.
Muraki K, Han L, Miller D, Murnane JP. Muraki K, et al. Nucleic Acids Res. 2015 Sep 18;43(16):7911-30. doi: 10.1093/nar/gkv714. Epub 2015 Jul 23. Nucleic Acids Res. 2015. PMID: 26209132 Free PMC article. - SMG6 regulates DNA damage and cell survival in Hippo pathway kinase LATS2-inactivated malignant mesothelioma.
Suzuki K, Tange M, Yamagishi R, Hanada H, Mukai S, Sato T, Tanaka T, Akashi T, Kadomatsu K, Maeda T, Miida T, Takeuchi I, Murakami H, Sekido Y, Murakami-Tonami Y. Suzuki K, et al. Cell Death Discov. 2022 Nov 5;8(1):446. doi: 10.1038/s41420-022-01232-w. Cell Death Discov. 2022. PMID: 36335095 Free PMC article. - Immune Activation Induces Telomeric DNA Damage and Promotes Short-Lived Effector T Cell Differentiation in Chronic HCV Infection.
Nguyen LN, Nguyen LNT, Zhao J, Schank M, Dang X, Cao D, Khanal S, Thakuri BKC, Zhang J, Lu Z, Wu XY, El Gazzar M, Ning S, Wang L, Moorman JP, Yao ZQ. Nguyen LN, et al. Hepatology. 2021 Nov;74(5):2380-2394. doi: 10.1002/hep.32008. Epub 2021 Aug 25. Hepatology. 2021. PMID: 34110660 Free PMC article. - Poly(ADP-ribosyl)ation in mammalian ageing.
Beneke S, Bürkle A. Beneke S, et al. Nucleic Acids Res. 2007;35(22):7456-65. doi: 10.1093/nar/gkm735. Epub 2007 Oct 2. Nucleic Acids Res. 2007. PMID: 17913748 Free PMC article. Review. - DNA damage in telomeres and mitochondria during cellular senescence: is there a connection?
Passos JF, Saretzki G, von Zglinicki T. Passos JF, et al. Nucleic Acids Res. 2007;35(22):7505-13. doi: 10.1093/nar/gkm893. Epub 2007 Nov 5. Nucleic Acids Res. 2007. PMID: 17986462 Free PMC article. Review.
References
- Alligood KJ, Milla M, Rhodes N, Ellis B, Kilpatrick KE, et al. Monoclonal antibodies generated against recombinant ATM support kinase activity. Hybridoma. 2000;19:317–321. - PubMed
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. - PubMed
- Baumann P, Cech TR. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science. 2001;292:1171–1175. - PubMed
- Broccoli D, Smogorzewska A, Chong L, de Lange T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat Genet. 1997;17:231–235. - PubMed
- Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM059413/GM/NIGMS NIH HHS/United States
- GM07739/GM/NIGMS NIH HHS/United States
- R01 GM049046/GM/NIGMS NIH HHS/United States
- GM59413/GM/NIGMS NIH HHS/United States
- R56 AG016642/AG/NIA NIH HHS/United States
- GM56888/GM/NIGMS NIH HHS/United States
- R37 GM049046/GM/NIGMS NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- R01 GM059413/GM/NIGMS NIH HHS/United States
- T32 GM007739/GM/NIGMS NIH HHS/United States
- R01 GM056888/GM/NIGMS NIH HHS/United States
- CA71387/CA/NCI NIH HHS/United States
- GM49046/GM/NIGMS NIH HHS/United States
- CA21765/CA/NCI NIH HHS/United States
- AG016642/AG/NIA NIH HHS/United States
- R01 CA071387/CA/NCI NIH HHS/United States
- R01 AG016642/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous