Acute cholesterol depletion impairs functional expression of tissue factor in fibroblasts: modulation of tissue factor activity by membrane cholesterol - PubMed (original) (raw)
Acute cholesterol depletion impairs functional expression of tissue factor in fibroblasts: modulation of tissue factor activity by membrane cholesterol
Samir K Mandal et al. Blood. 2005.
Abstract
Cholesterol, in addition to providing rigidity to the fluid membrane, plays a critical role in receptor function, endocytosis, recycling, and signal transduction. In the present study, we examined the effect of membrane cholesterol on functional expression of tissue factor (TF), a cellular receptor for clotting factor VIIa. Depletion of cholesterol in human fibroblasts (WI-38) with methyl-beta-cyclodextrin-reduced TF activity at the cell surface. Binding studies with radiolabeled VIIa and TF monoclonal antibody (mAB) revealed that reduced TF activity in cholesterol-depleted cells stems from the impairment of VIIa interaction with TF rather than the loss of TF receptors at the cell surface. Repletion of cholesterol-depleted cells with cholesterol restored TF function. Loss of caveolar structure on cholesterol removal is not responsible for reduced TF activity. Solubilization of cellular TF in different detergents indicated that a substantial portion of TF in fibroblasts is associated with noncaveolar lipid rafts. Cholesterol depletion studies showed that the TF association with these rafts is cholesterol dependent. Overall, the data presented herein suggest that membrane cholesterol functions as a positive regulator of TF function by maintaining TF receptors, probably in noncaveolar lipid rafts, in a high-affinity state for VIIa binding.
Figures
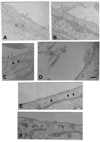
Figure 1. Ultrastructural localization of TF in fibroblasts and loss of caveolar structure on cholesterol depletion
Fibroblasts (WI-38 cells) were fixed and immunostained with TF mAb as described in “Materials and methods” (A–D). TF was localized on the cell membrane (A), on cellular processes (B), and in caveolae (C). The tip of a cellular process that was in contact with other cell/cellular process are stained densely for TF (D). Thin arrows point out gold particles, whereas arrowheads point out caveolae. Monolayers of WI-38 fibroblasts were treated with a control vehicle (E) or mβCD (10 mM) (F) for 45 minutes at 37°C. The cells were then fixed, sectioned, stained, and viewed under transmission electron microscope. Bar indicates 200 nm.
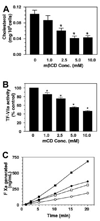
Figure 2. Effect of varying doses of cyclodextrin treatment on cholesterol depletion and TF-VIIa activity in fibroblasts
Monolayers of WI-38 cells were treated with varying doses (1 to 10 mM) of mβCD (A–B) for 45 minutes. At the end of 45 minutes, cells were washed and used for determining cholesterol content (A) or cell surface TF-VIIa activity by adding factor VIIa (10 nM) and factor X (175 nM) to the monolayers (B). (n = 4 to 6, mean ± SE). * denotes significantly differs (P < .05) from the control (untreated cells). Panel C depicts the time course of factor X activation by TF-VIIa in control and cholesterol-depleted cells (10 mM mβCD treatment for 45 minutes). Two different concentrations of factor X were used, 175 nM (circles) and 1 µM (squares). Filled symbols represent control cells, and open symbols represent mβCD-treated cells.
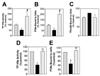
Figure 3. Effect of cholesterol depletion and cholesterol-loading on TF functional expression and reversibility of cholesterol effect
(A–C) Monolayers of WI-38 cells were treated for 45 minutes at 37°C with mβCD (10 mM) to deplete cholesterol or water-soluble cholesterol (mβCD-cholesterol, 1 mM) to load the cells with cholesterol. Then, the monolayers were incubated with (A) unlabeled VIIa (10 nM), followed by substrate factor X (175 nM) for 5 minutes at 37°C to measure TF functional activity; (B) 125I-VIIa (10 nM) or (C) 125I-TF mAB for 1 hour to measure VIIa or TF mAB binding to the cells. (D–E) Monolayers were first treated for 30 minutes at 37°C with mβCD (10 mM) to deplete cholesterol. After washing monolayers, cholesterol was reintroduced to the cells by incubating the cholesterol-depleted cells with cholesterol (1 mM): mβCD (10 mM) for 30 minutes. Following this, the cells were washed with buffer B and used to determine cell surface TF activity (D) and 125I-VIIa binding to cell surface TF (E) (n = 3, mean ± SE). * denotes significantly (P < .05) differs form the control; # denotes significantly (P < .05) differs from both the control and the cholesterol-depleted cells; and ** denotes significantly (P < .05) differs from mβCD-treated cells but not from the control. In A–C, ▩ indicates control; ■, cholesterol-deplete; ▨, cholesterol-laden. In D and E, ▩ indicates control; ■, mβCD; and ▨, mβCD plus cholesterol.
Figure 4. VIIa and TF mAB binding to cholesterol-depleted cells
Control and cholesterol-depleted cells were incubated with varying concentration of 125I-VIIa in the presence and absence of anti-TF IgG (A) or 125I-TF mAB (B) for 2 hours at 4°C. At the end of the 2-h incubation, the unbound ligands were removed, cells were washed, and the cell-associated radioactivity was counted. Specific VIIa binding, shown in panel A, was determined by subtracting the nonspecific binding (VIIa binding to cells in the presence of anti-TF IgG) from the total binding (VIIa binding in the absence of anti-TF IgG) (n = 4 to 6, mean ± SE).
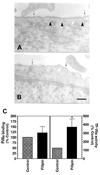
Figure 5. Effect of filipin on caveolar structure and TF expression
Monolayers of WI-38 fibroblasts were treated with a control vehicle (A) or filipin (5 µg/mL) (B) for 15 minutes at 37°C. Cells were fixed, sectioned, stained for TF by immunogold, and visualized under transmission electron microscope. Thin arrows point out gold particles whereas arrowheads point out caveolae. Bar indicates 200 nm. (C) Control or filipin-treated monolayers were incubated with either 125I-VIIa (10 nM) or unlabeled VIIa (10 nM) and factor X (175 nM) to determine VIIa binding and TF-VIIa activity (n = 4, mean ± SE). * denotes significantly differs (P < .05) from the control.
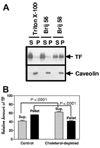
Figure 6. Tissue factor is associated with Brij 58 detergent-resistant, cholesterol-based membrane domains
(A). WI-38 cells were lysed in 0.5% of the indicated detergent for 30 minutes at 4°C, and the postnuclear supernatants were fractionated into pellets (P) and supernatants (S) by centrifugation at 16 000_g_ for 30 minutes and analyzed by immunoblotting. (B). WI-38 cells were cholesterol-depleted with mβCD (10 mM for 30 minutes) at 37°C and subsequently lysed in Brij 58. The samples were analyzed for TF by Western blotting, and the signals were quantitated by densitometry (n = 5, mean ± SE).
Similar articles
- Cellular localization and trafficking of tissue factor.
Mandal SK, Pendurthi UR, Rao LV. Mandal SK, et al. Blood. 2006 Jun 15;107(12):4746-53. doi: 10.1182/blood-2005-11-4674. Epub 2006 Feb 21. Blood. 2006. PMID: 16493004 Free PMC article. - Modulation of tissue factor-factor VIIa signaling by lipid rafts and caveolae.
Awasthi V, Mandal SK, Papanna V, Rao LV, Pendurthi UR. Awasthi V, et al. Arterioscler Thromb Vasc Biol. 2007 Jun;27(6):1447-55. doi: 10.1161/ATVBAHA.107.143438. Epub 2007 Apr 5. Arterioscler Thromb Vasc Biol. 2007. PMID: 17413039 Free PMC article. - Binding of factor VIIa to tissue factor induces alterations in gene expression in human fibroblast cells: up-regulation of poly(A) polymerase.
Pendurthi UR, Alok D, Rao LV. Pendurthi UR, et al. Proc Natl Acad Sci U S A. 1997 Nov 11;94(23):12598-603. doi: 10.1073/pnas.94.23.12598. Proc Natl Acad Sci U S A. 1997. PMID: 9356495 Free PMC article. - Regulation of tissue factor-factor VIIa expression on cell surfaces: a role for tissue factor-factor VIIa endocytosis.
Rao LV, Pendurthi UR. Rao LV, et al. Mol Cell Biochem. 2003 Nov;253(1-2):131-40. doi: 10.1023/a:1026004208822. Mol Cell Biochem. 2003. PMID: 14619963 Review. - Tissue factor-dependent factor VIIa signaling.
Petersen LC, Freskgård P, Ezban M. Petersen LC, et al. Trends Cardiovasc Med. 2000 Feb;10(2):47-52. doi: 10.1016/s1050-1738(00)00041-4. Trends Cardiovasc Med. 2000. PMID: 11150729 Review.
Cited by
- Tissue factor: mechanisms of decryption.
Rao LV, Kothari H, Pendurthi UR. Rao LV, et al. Front Biosci (Elite Ed). 2012 Jan 1;4(4):1513-27. doi: 10.2741/477. Front Biosci (Elite Ed). 2012. PMID: 22201972 Free PMC article. Review. - The role of procoagulant phospholipids on the surface of circulating blood cells in thrombosis and haemostasis.
Protty MB, Jenkins PV, Collins PW, O'Donnell VB. Protty MB, et al. Open Biol. 2022 Apr;12(4):210318. doi: 10.1098/rsob.210318. Epub 2022 Apr 20. Open Biol. 2022. PMID: 35440201 Free PMC article. Review. - Cellular localization and trafficking of tissue factor.
Mandal SK, Pendurthi UR, Rao LV. Mandal SK, et al. Blood. 2006 Jun 15;107(12):4746-53. doi: 10.1182/blood-2005-11-4674. Epub 2006 Feb 21. Blood. 2006. PMID: 16493004 Free PMC article. - Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation.
Reinhardt C, von Brühl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Reinhardt C, et al. J Clin Invest. 2008 Mar;118(3):1110-22. doi: 10.1172/JCI32376. J Clin Invest. 2008. PMID: 18274674 Free PMC article. - Role of Tissue Factor in the Pathogenesis of COVID-19 and the Possible Ways to Inhibit It.
Cañas CA, Cañas F, Bautista-Vargas M, Bonilla-Abadía F. Cañas CA, et al. Clin Appl Thromb Hemost. 2021 Jan-Dec;27:10760296211003983. doi: 10.1177/10760296211003983. Clin Appl Thromb Hemost. 2021. PMID: 33784877 Free PMC article. Review.
References
- Bloch K. Cholesterol: evolution of structure and function. In: Vance DE, Vance J, editors. Biochemistry of Lipids, Lipoproteins and Membranes. Amsterdam: Elsevier Science; 1991. pp. 363–381.
- Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. - PubMed
- Fielding CJ, Fielding PE. Cholesterol and caveolae: structural and functional relationships. Biochim Biophys Acta. 2000;1529:210–222. - PubMed
- Schlegel A, Lisanti MP. The caveolin triad: caveolae biogenesis, cholesterol trafficking, and signal. Cytokine Growth Factor Rev. 2001;12:41–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL058869-02/HL/NHLBI NIH HHS/United States
- R01 HL065500-02/HL/NHLBI NIH HHS/United States
- R01 HL065500/HL/NHLBI NIH HHS/United States
- R01 HL058869-03/HL/NHLBI NIH HHS/United States
- R01 HL058869/HL/NHLBI NIH HHS/United States
- HL58869/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous