A supertree approach to shorebird phylogeny - PubMed (original) (raw)
A supertree approach to shorebird phylogeny
Gavin H Thomas et al. BMC Evol Biol. 2004.
Abstract
Background: Order Charadriiformes (shorebirds) is an ideal model group in which to study a wide range of behavioural, ecological and macroevolutionary processes across species. However, comparative studies depend on phylogeny to control for the effects of shared evolutionary history. Although numerous hypotheses have been presented for subsets of the Charadriiformes none to date include all recognised species. Here we use the matrix representation with parsimony method to produce the first fully inclusive supertree of Charadriiformes. We also provide preliminary estimates of ages for all nodes in the tree.
Results: Three main lineages are revealed: i) the plovers and allies; ii) the gulls and allies; and iii) the sandpipers and allies. The relative position of these clades is unresolved in the strict consensus tree but a 50% majority-rule consensus tree indicates that the sandpiper clade is sister group to the gulls and allies whilst the plover group is placed at the base of the tree. The overall topology is highly consistent with recent molecular hypotheses of shorebird phylogeny.
Conclusion: The supertree hypothesis presented herein is (to our knowledge) the only complete phylogenetic hypothesis of all extant shorebirds. Despite concerns over the robustness of supertrees (see Discussion), we believe that it provides a valuable framework for testing numerous evolutionary hypotheses relating to the diversity of behaviour, ecology and life-history of the Charadriiformes.
Figures
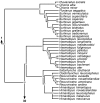
Figure 1
Previous hypotheses shorebird phylogeny. Family and subfamily level relationships of shorebirds based on: a) Morphological data [19]; b) DNA-DNA hybridisation [16]; c) Sequence analysis of RAG-1 [20, 21], cytochrome-b [22] and myoglobin intron II [21].
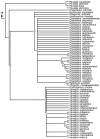
Figure 2
Summary of shorebird supertree. Family and subfamily level relationships of shorebirds based on 50% majority rule tree. Numbers on nodes refer to age estimates in additional file 1. Boxed node numbers indicate that node collapses to its immediate ancestor in the strict consensus tree (see also additional files 2 and 3 for the full 50% majority rule and strict consensus trees respectively).
Figure 3
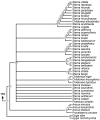
Phylogeny of Larini. 50% majority rule supertree showing the relationships of the Larini. Numbers on nodes refer to age estimates in additional file 1. Boxed node numbers indicate that node collapses to its immediate ancestor in the strict consensus tree (see also additional files 2 and 3 for the full 50% majority rule and strict consensus trees respectively).
Figure 4
Phylogeny of Sternini. 50% majority rule supertree showing the relationships of the Sternini. Numbers on nodes refer to age estimates in additional file 1. Boxed node numbers indicate that node collapses to its immediate ancestor in the strict consensus tree (see also additional files 2 and 3 for the full 50% majority rule and strict consensus trees respectively).
Figure 5
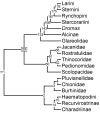
Phylogeny of Rynchopini, Stercorariini, Dromas, Alcinae, and Glareolidae 50% majority rule supertree showing the relationships of the Rynchopini, Stercorariini, Dromas, Alcinae, and Glareolidae. Numbers on nodes refer to age estimates in additional file 1. Boxed node numbers indicate that node collapses to its immediate ancestor in the strict consensus tree (see also additional files 2 and 3 for the full 50% majority rule and strict consensus trees respectively). Node numbers 139 and 140 have no support from any source tree and are novel clades.
Figure 6
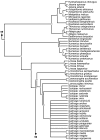
Phylogeny of Jacanidae, Rostratulidae, Thinocoridae, Pedionomidae and Scolopacidae 50% majority rule supertree showing the relationships of the Jacanidae, Rostratulidae, Thinocoridae, Pedionomidae and Scolopacidae. Numbers on nodes refer to age estimates in additional file 1. Boxed node numbers indicate that node collapses to its immediate ancestor in the strict consensus tree (see also additional files 2 and 3 for the full 50% majority rule and strict consensus trees respectively). Node numbers 85 and 89 have no support from any source tree and are novel clades.
Figure 7
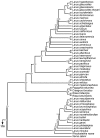
Phylogeny of Scolopacidae 50% majority rule supertree showing the relationships of the Scolopacidae. Numbers on nodes refer to age estimates in additional file 1. Boxed node numbers indicate that node collapses to its immediate ancestor in the strict consensus tree (see also additional files 2 and 3 for the full 50% majority rule and strict consensus trees respectively). Node numbers 108 and 122 have no support from any source tree and are novel clades.
Figure 8
Phylogeny of Pluvianellidae, Chionidae, Burhinidae, Haematopodini and Recurvirostrini 50% majority rule supertree showing the relationships of the Pluvianellidae, Chionidae, Burhinidae, Haematopodini and Recurvirostrini. Numbers on nodes refer to age estimates in additional file 1. Boxed node numbers indicate that node collapses to its immediate ancestor in the strict consensus tree (see also additional files 2 and 3 for the full 50% majority rule and strict consensus trees respectively). Node numbers 20 and 29 have no support from any source tree and are novel clades.
Figure 9
Phylogeny Charadriinae 50% majority rule supertree showing the relationships of the Charadriinae. Numbers on nodes refer to age estimates in additional file 1. Boxed node numbers indicate that node collapses to its immediate ancestor in the strict consensus tree (see also additional files 2 and 3 for the full 50% majority rule and strict consensus trees respectively). Node number 57 have no support from any source tree and are novel clades.
Similar articles
- Phylogenetic relationships and divergence times of Charadriiformes genera: multigene evidence for the Cretaceous origin of at least 14 clades of shorebirds.
Baker AJ, Pereira SL, Paton TA. Baker AJ, et al. Biol Lett. 2007 Apr 22;3(2):205-9. doi: 10.1098/rsbl.2006.0606. Biol Lett. 2007. PMID: 17284401 Free PMC article. - Comprehensive taxon sampling and vetted fossils help clarify the time tree of shorebirds (Aves, Charadriiformes).
Černý D, Natale R. Černý D, et al. Mol Phylogenet Evol. 2022 Dec;177:107620. doi: 10.1016/j.ympev.2022.107620. Epub 2022 Aug 28. Mol Phylogenet Evol. 2022. PMID: 36038056 - Inter-familial relationships of the shorebirds (Aves: Charadriiformes) based on nuclear DNA sequence data.
Ericson PG, Envall I, Irestedt M, Norman JA. Ericson PG, et al. BMC Evol Biol. 2003 Jul 23;3:16. doi: 10.1186/1471-2148-3-16. Epub 2003 Jul 23. BMC Evol Biol. 2003. PMID: 12875664 Free PMC article. - Genus-level supertree of Cyprinidae (Actinopterygii: Cypriniformes), partitioned qualitative clade support and test of macro-evolutionary scenarios.
Gaubert P, Denys G, Oberdorff T. Gaubert P, et al. Biol Rev Camb Philos Soc. 2009 Nov;84(4):653-89. doi: 10.1111/j.1469-185X.2009.00091.x. Biol Rev Camb Philos Soc. 2009. PMID: 19857213 Review. - A phylogenetic supertree of the bats (Mammalia: Chiroptera).
Jones KE, Purvis A, MacLarnon A, Bininda-Emonds OR, Simmons NB. Jones KE, et al. Biol Rev Camb Philos Soc. 2002 May;77(2):223-59. doi: 10.1017/s1464793101005899. Biol Rev Camb Philos Soc. 2002. PMID: 12056748 Review.
Cited by
- Genome skimming identifies polymorphism in tern populations and species.
Jackson DG, Emslie SD, van Tuinen M. Jackson DG, et al. BMC Res Notes. 2012 Feb 14;5:94. doi: 10.1186/1756-0500-5-94. BMC Res Notes. 2012. PMID: 22333071 Free PMC article. - Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. II. Analysis and discussion.
Livezey BC, Zusi RL. Livezey BC, et al. Zool J Linn Soc. 2007 Jan 1;149(1):1-95. doi: 10.1111/j.1096-3642.2006.00293.x. Zool J Linn Soc. 2007. PMID: 18784798 Free PMC article. - Evolution of within-colony distribution patterns of birds in response to habitat structure.
Minias P. Minias P. Behav Ecol Sociobiol. 2014;68(5):851-859. doi: 10.1007/s00265-014-1697-8. Epub 2014 Mar 4. Behav Ecol Sociobiol. 2014. PMID: 24771961 Free PMC article. - Egg characteristics vary longitudinally in Arctic shorebirds.
Liu J, Chai Z, Wang H, Ivanov A, Kubelka V, Freckleton R, Zhang Z, Székely T. Liu J, et al. iScience. 2023 May 19;26(6):106928. doi: 10.1016/j.isci.2023.106928. eCollection 2023 Jun 16. iScience. 2023. PMID: 37305692 Free PMC article.
References
- Monroe BL, Jr, Sibley CG. A World Checklist of Birds. New Haven; Yale University Press; 1993.
- Whitfield DP, Tomkovich PS. Mating system and timing of breeding in Holarctic waders. Biol J Linn Soc. 1996;57:277–290. doi: 10.1006/bijl.1996.0015. - DOI
- Reynolds JD, Székely T. The evolution of parental care in shorebirds: life histories, ecology, and sexual selection. Behav Ecol. 1997;8:126–134.
- Borowik OA, Mclennan DA. Phylogenetic patterns of parental care in Calidrine sandpipers. Auk. 1999;116:1107–1117.
- Figuerola J. A comparative study on the evolution of reversed size dimorphism in monogamous waders. Biol J Linn Soc. 1999;67:1–18. doi: 10.1006/bijl.1998.0254. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources