Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice - PubMed (original) (raw)
Comparative Study
Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice
Weifang Rong et al. J Physiol. 2004.
Abstract
The aim of this study was to investigate the contribution of the TRPV1 receptor to jejunal afferent sensitivity in the murine intestine. Multiunit activity was recorded in vitro from mesenteric afferents supplying segments of mouse jejunum taken from wild-type (WT) and TRPV1 knockout (TRPV1(-/-)) animals. In WT preparations, ramp distension of the gut (up to 60 mmHg) produced biphasic changes in afferent activity so the pressure-response curve had an initial rapid increase in afferent discharge followed by a second phase of slower increase in activity. Afferent response to distension was significantly lower in TRPV1(-/-) than in WT mice. Single-unit analysis revealed three functional types of afferent fibres: (1) low-threshold fibres (2) wide dynamic range fibres and (3) high-threshold fibres. There was a marked downward shift of the pressure-response curve for wide dynamic range fibres in the TRPV1(-/-) mice as compared to the WT controls. The afferent response to intraluminal hydrochloric acid (20 mM) was also attenuated in the TRPV1(-/-) mice. In contrast, the response to bath application of bradykinin (1 microm, 3 ml) was not significantly different between the two groups. The TRPV1 antagonist capsazepine (10 microm) significantly attenuated the nerve responses to distension, intraluminal acid and bradykinin, as well as the spontaneous discharge in WT mice. The WT jejunal afferents responded to capsaicin with rapid increases in afferent activity, whereas TRPV1(-/-) afferents were not at all sensitive to capsaicin. Previous evidence indicates that TRPV1 is not mechanosensitive, so the results of the present study suggest that activation of TRPV1 may sensitize small intestinal afferent neurones.
Figures
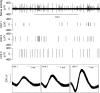
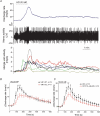
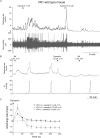
Figure 1. Spontaneous mesenteric nerve activity in the mouse in vitro jejunum preparation
Top trace, typical whole-nerve signal with multiple action potentials of different size and shape. Single-unit activity can be discriminated based on the spike waveform as is illustrated in the bottom panels in which the action potentials of three units are superimposed in separate templates (Units 1–3). Middle traces (Units 1–3) are the waveform of each single unit.
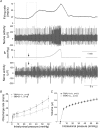
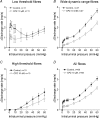
Figure 2. Mesenteric afferent responses to jejunal distension in wild-type (WT) and TRPV1−/− mice
A, representative recording of distension-induced biphasic changes in afferent activity in a WT preparation. The bottom trace is an expanded view of the nerve activity around the time when distension started. Spikes of larger amplitudes are truncated in order to illustrate spikes of lower amplitudes, which tend to be low-threshold fibres firing vigorously at low distension pressure. B, pressure–response relationships of multiunit activity in 19 WT and 18 TRPV1−/− preparations. The curve is biphasic and the second phase can be further divided into two components (between 2 and 20 mmHg and between 20 and 60 mmHg). There are significant differences in the pressure–response curves between the WT and the TRPV1−/− groups (ANCOVA, see text for detail). C, relationships between perfusion volume and intraluminal pressure of the jejunal segments are not different between the WT and the TRPV1−/− groups.
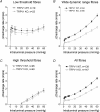
Figure 3. Single-unit responses to jejunal distensions in WT and the TRPV1−/− preparations
A_–_C, pressure–response curves of three different functional types of fibres: low-threshold fibres (LT fibres), wide dynamic range fibres (WDR fibres) and high-threshold fibres (HT fibres). D, pressure–response curves of all fibres. Note that the curves for WDR fibres are significantly different between WT and TRPV1−/− mice, but the curves for LT and HT fibres are not (ANCOVA).
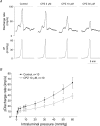
Figure 4. The effects of capsazepine (CPZ) on distension-induced mesenteric afferent activity in TRPV1 WT mice
A, an example of changes in afferent discharge rate in response to increases in intraluminal pressure in control and in the presence of different concentrations of CPZ. This was a continuous recording in a TRPV1 WT preparation, but the traces are truncated for clarity of illustration. B, the pressure–multiunit response relationships in control and in the presence of CPZ 10 μ
m
. The two curves are significantly different (ANCOVA).
Figure 5. The effects of CPZ on single-unit responses to jejunal distensions
A_–_C, pressure–response relationships of LT fibres, WDR fibres and HT fibres in control and in the presence of CPZ 10 μ
m
. D, plots of the pressure–response curves of all single units averaged. CPZ resulted in a significant shift of the pressure–response curves of all three fibre types (ANCOVA).
Figure 6. The responses of jejunal afferents to intraluminal infusion of HCl in the WT and the TRPV1−/− mice
A, representative recording of the multiunit activity following intraluminal infusion of HCl (20 m
m
), with the bottom trace showing changes of the activity in six single units. B, comparison of the multiunit rate histogram following intraluminal acid infusion in WT (n = 12) and TRPV1−/− preparations (n = 8). *P < 0.05 and **P < 0.01, Student's t test. C, the rate histogram of whole nerves following acid infusion in control and in the presence of CPZ in WT mice. *P < 0.05 and **P < 0.01, paired t test.
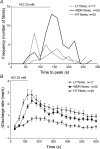
Figure 7. The response of single units in mesenteric nerves to intraluminal acid in TRVP1 WT mice
A, frequency histogram of time to peak (the latency from the start of acid infusion to single-unit peak activity) in different functional types of fibres. The Kruskal–Wallis test indicates that HT fibres reached peak activity significantly faster than WDR fibres and LT fibres. B, the average rate histogram of different types of afferent fibres following application. Note that the response in LT fibres is less than in WDR and HT fibres.
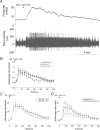
Figure 8. The responses of mesenteric afferent nerve to bradykinin in WT and TRPV1−/− mice
A, representative recording of the multiunit afferent activity in response to bath application of bradykinin (BK 1 μ
m
, 3 ml). B, the average-rate histogram of different types of afferent fibres following application of BK. C, comparison of the averaged level of increases in multiunit activity following application of BK (1 μ
m
, 3 ml) in WT (n = 13) and in TRPV1−/− preparations (n = 14). No significant difference was found between the two groups (Student's t test). D, the effects of CPZ on the multiunit responses to BK in seven TRPV1 WT preparations. *P < 0.05 and **P < 0.01, paired t test.
Figure 9. The responses of mesenteric afferent nerves to capsaicin in WT and TRPV1−/− mice
A, upon administration of 1 μ
m
capsaicin in WT mice, there was an excitation on mesenteric afferents. B, following 10 μ
m
capsaicin, there was a brief phase of intense discharge followed by a decrease in spontaneous nerve firing and reduced responsiveness to BK, intraluminal acid (not shown) and distension, which is indicative of afferent nerve desensitization. C, cumulative data showing changes in afferent activity following application of capsaicin in WT preparations. Note that TRPV1 KO preparations failed to respond to capsaicin.
Similar articles
- Spontaneous hypersensitivity in mesenteric afferent nerves of mice deficient in the sst2 subtype of somatostatin receptor.
Rong W, Winchester WJ, Grundy D. Rong W, et al. J Physiol. 2007 Jun 1;581(Pt 2):779-86. doi: 10.1113/jphysiol.2006.125187. Epub 2007 Mar 15. J Physiol. 2007. PMID: 17363388 Free PMC article. - Bladder afferent sensitivity in wild-type and TRPV1 knockout mice.
Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Daly D, et al. J Physiol. 2007 Sep 1;583(Pt 2):663-74. doi: 10.1113/jphysiol.2007.139147. Epub 2007 Jul 12. J Physiol. 2007. PMID: 17627983 Free PMC article. - Purinergic contribution to small intestinal afferent hypersensitivity in a murine model of postinfectious bowel disease.
Rong W, Keating C, Sun B, Dong L, Grundy D. Rong W, et al. Neurogastroenterol Motil. 2009 Jun;21(6):665-71, e32. doi: 10.1111/j.1365-2982.2008.01259.x. Epub 2009 Feb 11. Neurogastroenterol Motil. 2009. PMID: 19220757 - The unsilent majority-TRPV1 drives "spontaneous" transmission of unmyelinated primary afferents within cardiorespiratory NTS.
Andresen MC, Hofmann ME, Fawley JA. Andresen MC, et al. Am J Physiol Regul Integr Comp Physiol. 2012 Dec 15;303(12):R1207-16. doi: 10.1152/ajpregu.00398.2012. Epub 2012 Oct 17. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 23076872 Free PMC article. Review. - TRPV channels as temperature sensors.
Benham CD, Gunthorpe MJ, Davis JB. Benham CD, et al. Cell Calcium. 2003 May-Jun;33(5-6):479-87. doi: 10.1016/s0143-4160(03)00063-0. Cell Calcium. 2003. PMID: 12765693 Review.
Cited by
- Identifying the Ion Channels Responsible for Signaling Gastro-Intestinal Based Pain.
Brierley SM, Hughes PA, Harrington AM, Rychkov GY, Blackshaw LA. Brierley SM, et al. Pharmaceuticals (Basel). 2010 Aug 26;3(9):2768-2798. doi: 10.3390/ph3092768. Pharmaceuticals (Basel). 2010. PMID: 27713376 Free PMC article. Review. - Spontaneous hypersensitivity in mesenteric afferent nerves of mice deficient in the sst2 subtype of somatostatin receptor.
Rong W, Winchester WJ, Grundy D. Rong W, et al. J Physiol. 2007 Jun 1;581(Pt 2):779-86. doi: 10.1113/jphysiol.2006.125187. Epub 2007 Mar 15. J Physiol. 2007. PMID: 17363388 Free PMC article. - Activation of TREK currents by riluzole in three subgroups of cultured mouse nodose ganglion neurons.
Fernández-Fernández D, Cadaveira-Mosquera A, Rueda-Ruzafa L, Herrera-Pérez S, Veale EL, Reboreda A, Mathie A, Lamas JA. Fernández-Fernández D, et al. PLoS One. 2018 Jun 21;13(6):e0199282. doi: 10.1371/journal.pone.0199282. eCollection 2018. PLoS One. 2018. PMID: 29928032 Free PMC article. - Depolarizing Effectors of Bradykinin Signaling in Nociceptor Excitation in Pain Perception.
Choi SI, Hwang SW. Choi SI, et al. Biomol Ther (Seoul). 2018 May 1;26(3):255-267. doi: 10.4062/biomolther.2017.127. Biomol Ther (Seoul). 2018. PMID: 29378387 Free PMC article. Review. - TRPV channels and vascular function.
Baylie RL, Brayden JE. Baylie RL, et al. Acta Physiol (Oxf). 2011 Sep;203(1):99-116. doi: 10.1111/j.1748-1716.2010.02217.x. Epub 2010 Dec 9. Acta Physiol (Oxf). 2011. PMID: 21062421 Free PMC article. Review.
References
- Accarino AM, Azpiroz F, Malagelada JR. Selective dysfunction of mechanosensitive intestinal afferents in irritable bowel syndrome. Gastroenterology. 1995;108:636–643. - PubMed
- Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acid-sensing pathways of rat duodenum. Am J Physiol Gastrointest Liver Physiol. 1999;277:G268–274. - PubMed
- Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990;31:191–201. - PubMed
- Blackshaw LA, Page AJ, Partosoedarso ER. Acute effects of capsaicin on gastrointestinal vagal afferents. Neuroscience. 2000;96:407–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases