Activation of chaperone-mediated autophagy during oxidative stress - PubMed (original) (raw)
Activation of chaperone-mediated autophagy during oxidative stress
Roberta Kiffin et al. Mol Biol Cell. 2004 Nov.
Abstract
Oxidatively damaged proteins accumulate with age in almost all cell types and tissues. The activity of chaperone-mediated autophagy (CMA), a selective pathway for the degradation of cytosolic proteins in lysosomes, decreases with age. We have analyzed the possible participation of CMA in the removal of oxidized proteins in rat liver and cultured mouse fibroblasts. Added to the fact that CMA substrates, when oxidized, are more efficiently internalized into lysosomes, we have found a constitutive activation of CMA during oxidative stress. Oxidation-induced activation of CMA correlates with higher levels of several components of the lysosomal translocation complex, but in particular of the lumenal chaperone, required for substrate uptake, and of the lysosomal membrane protein (lamp) type 2a, previously identified as a receptor for this pathway. In contrast with the well characterized mechanism of CMA activation during nutritional stress, which does not require de novo synthesis of the receptor, oxidation-induced activation of CMA is attained through transcriptional up-regulation of lamp2a. We conclude that CMA is activated during oxidative stress and that the higher activity of this pathway under these conditions, along with the higher susceptibility of the oxidized proteins to be taken up by lysosomes, both contribute to the efficient removal of oxidized proteins.
Figures
Figure 2.
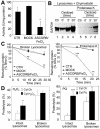
Oxidation of CMA substrates facilitates their uptake by lysosomes. (A) GAPDH was exposed to an ascorbic acid/iron oxidizing reaction for 30 min as described under Materials and Methods. The remaining specific enzymatic activity of untreated GAPDH (CTR) and of GAPDH exposed to the oxidant mixture (ASCORB/FeCl3) or to the dialysis buffer only (MOCK) is shown. Values are mean ± SE of two different preparations with triplicate measurements. (B) Unmodified GAPDH (Ctr) or GAPDH exposed to the oxidant mixture for the indicated periods of time was incubated for 30 min under standard conditions with intact chymostatin-treated liver lysosomes isolated from 20-h starved rats. Where indicated, samples were treated with proteinase K to remove GAPDH bound to the cytosolic side of the lysosomal membrane. At the end of the incubation, lysosomes were collected by centrifugation and subjected to SDS-PAGE and immunoblot for GAPDH. (C) The different forms of GAPDH (50 μg) described in A were incubated with lysosomal enzymes (25 μg of protein of broken lysosomes; pH 4.5) (left) or proteinase K (0.1 μg) (right) at 37 or 0°C, respectively. At the indicated times aliquots were taken and subjected to SDS-PAGE and Coomassie Blue staining. Values are the mean + SE of the densitometric values for GAPDH from three different experiments. (D) Mouse fibroblasts were metabolically labeled with [3H]leucine for 48 h. A group of fibroblasts was exposed to 100 μM H2O2 (left) or 40 μM paraquat (Pq) for 1 h during the labeling. After cavitation, cytosolic fractions (Cyt) from untreated (Ctr) and H2O2 or paraquat-treated fibroblasts were prepared and incubated for 30 min at 37°C with intact rat liver lysosomes (25 μg of protein) or with lysosomes disrupted by a hypotonic shock (broken lysosomes; 15 μg of protein) under standard conditions. At the end of the incubation proteolysis was calculated as the amount of acid precipitable radioactivity (protein) transformed in acid soluble (amino acids and small peptides). Values are the mean + SE of six different experiments (*p < 0.05, **p < 0.01, ***p < 0.001).
Figure 1.
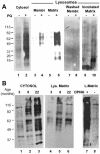
Oxidized proteins in lysosomes active for CMA. (A) Cytosol (20 μg of protein) and lysosomal membranes and matrices (10 μg of protein) separated after hypotonic shock from lysosomes enriched in hsc70 isolated from livers of untreated or paraquat (PQ)-treated rats were derivatized and subjected to SDS-PAGE and immunoblot against 2,4-dinitrophenylhydrazine (DPNH) moieties. To show the protein pattern in all lanes, exposure of lanes 1 and 2 was 5 times shorter than for the other lanes. Lanes 7 and 8 are duplicate samples of lanes 3 and 4, in which membranes were washed with 1 M NaCl before derivatization. Lanes 9 and 10 are duplicate samples of lanes 5 and 6 in which the matrices were incubated for 20 min at 37°C before derivatization. No bands were detected in the same samples when derivatization was omitted (our unpublished data). (B) Cytosol (40 μg of protein) and lysosomal matrices (20 μg of protein) were isolated from livers of 3-, 9- and 22-mo-old rats. Content of oxidized proteins in these fractions was analyzed as in A. Exposure of lanes 1–3 was 3 times shorter than for lanes 4–6. Lanes 7 and 8 show the same sample than lane 5 derivatized or not with DPNH and immunobloted as the others. The film is overexposed (8 times lane 5 exposure) to show the low content of protein bands nonspecifically recognized by the antibody.
Figure 3.
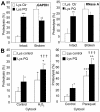
Additive effect of mild oxidative stress on CMA substrate proteins and on lysosomes. (A) Degradation of [14C]GAPDH (left) and [14C]RNase A (right) by lysosomes isolated from rats treated with paraquat as described under Materials and Methods. Incubations were carried out as described in Fig. 2D. Values are the mean + SE of five to eight different experiments (*p < 0.05, **p < 0.01, ***p < 0.001). (B) Degradation of radiolabeled cytosolic proteins prepared as in Figure 2D from untreated fibroblasts (control) or from fibroblasts exposed to H2O2 (left) or paraquat (right), by intact liver lysosomes from untreated rats (Lysosomes control) or from rats treated with paraquat (Lysosomes PQ) as described under Materials and Methods. Proteolysis was calculated as in Figure 2D. Values are mean + SE of six different experiments. *, differences compared with lysosomes control; +, differences compared with cytosol control; †, differences comparing lysosomes and cytosol control, to lysosomes and cytosol treated (*p < 0.05, **p < 0.01, ***p < 0.001).
Figure 4.
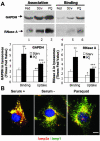
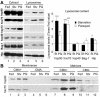
Activation of CMA during mild oxidative stress. (A) Association and binding of GAPDH and RNase A to intact lysosomes from untreated fed rats, 48-h starved rats (Strv) or fed rats treated with paraquat (PQ). Lysosomes in lanes 1–3 were preincubated with chymostatin to prevent protein degradation. Incubations were carried out in an isotonic buffer, as described under Materials and Methods, and levels of associated proteins were determined by immunoblot of the lysosomes collected by centrifugation. Bottom: densitometric quantification (mean + SE) of 4–6 immunoblots similar to the ones shown here. Values are expressed as the fold of increase in binding and in uptake (association-binding) for the lysosomes from starved or paraquat-treated rats, compared with lysosomes from untreated fed animals. *, differences compared with fed animals; +, differences compared with starved animals (*p < 0.1, **p < 0.05, ***p < 0.01) (B) Immunofluorescence for lamp2a (red) and lamp1 (green) in mouse fibroblasts after removing the serum from the culture medium (serum-) or after treatment with paraquat in cells maintained in serum supplemented medium. The merged image of both fluorochrome channels is shown. Bar, 50 μm.
Figure 5.
Changes in lysosome-associated chaperones during mild oxidative stress. (A) Cytosol and lysosomes (75 μg of protein) isolated from normally fed, 48-h starved rats (Stv), or fed rats treated with paraquat (PQ) as labeled were subjected to SDS-PAGE and immunoblot for the indicated proteins. Right, densitometric quantification (mean + SE) of the chaperones in the lysosomal fraction in four immunoblots similar to the ones shown here. Levels in lysosomes from fed animals were given an arbitrary value of 1 (dotted line). *, differences compared with fed animals (*p < 0.1, **p < 0.05, ***p < 0.01). (B) Lysosomes with high (CMA+) and low (CMA-) activity for CMA (Cuervo et al., 1997) were isolated from the same groups of animals as detailed in A. After hypotonic shock and centrifugation, membranes and matrices (50 μg of protein) were separated and subjected to SDS-PAGE and immunoblot for hsc90 (top) or hsc70 (bottom).
Figure 6.
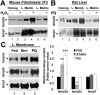
Lamp2a levels at the lysosomal membrane increase during mild oxidative stress. (A and B) Immunoblot for lamp2a and lamp1 of homogenates (50 μg of protein), lysosomal membranes (L. Memb) and lysosomal matrices (L. Matrix) (10 μg of protein) isolated from cultured mice fibroblasts, exposed or not to 100 μM H2O2 (A) or from liver of rats treated or not with paraquat (PQ). (C) Imunoblot for lamp2a, lamp2c, and lamp1 of lysosomal membranes isolated from livers of fed rats, 48-h starved (Strv) rats, or fed rats treated with paraquat (PQ). Right, densitometric quantification of six to eight immunoblots as the ones shown here. Values are expressed as fold of the values in fed untreated animals and are the mean + SE of six different experiments. Levels in lysosomes from fed animals were given an arbitrary value of 1. *, differences compared with fed animals (*p < 0.05, **p < 0.01, ***p < 0.001).
Figure 7.
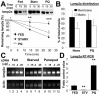
A novel mechanism for activation of CMA during mild oxidative stress. (A) Degradation of lamp2a in isolated membranes from normally fed rats, 48-h starved rats, or fed rats treated with paraquat (PQ), as indicated under Materials and Methods. Degradation of lamp2a was followed by immunoblot of the lysosomal membranes with an antibody specific against its cytosolic tail, at different times of the incubation. Values are the mean + SD of the densitometric quantification of immunoblots from four different experiments. A representative immunoblot is shown in the top insets. (B) Distribution of lamp2a between the lysosomal membrane and the matrix in lysosomes isolated from normally fed rats, treated or not with PQ. Values are mean + SE of the densitometric quantification of four immunoblots similar to the one shown in Figure 6B. (C) A 120-nucleotide fragment of lamp2a and a 108-nucleotide fragment of actin were amplified, through PCR, from increasing concentrations of total mRNA isolated from the livers of normally fed, 48-h starved, or paraquat-treated fed rats. The electrophoretic pattern of the products in a 2% agarose gel is shown. (D) Semiquantitative real-time PCR was used to compare mRNA expression levels for lamp2a in the same samples as detailed in C. Values were corrected for actin amplification in each samples and are expressed as fold increase compared to mRNA lamp2a values in fed untreated rats (that was given an arbitrary value of 1). Values are mean + SD for four different experiments. *, differences compared with fed animals (**p < 0.01, ***p < 0.001).
Similar articles
- Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age.
Kiffin R, Kaushik S, Zeng M, Bandyopadhyay U, Zhang C, Massey AC, Martinez-Vicente M, Cuervo AM. Kiffin R, et al. J Cell Sci. 2007 Mar 1;120(Pt 5):782-91. doi: 10.1242/jcs.001073. Epub 2007 Feb 6. J Cell Sci. 2007. PMID: 17284523 - Chaperone mediated autophagy in aging: Starve to prosper.
Xilouri M, Stefanis L. Xilouri M, et al. Ageing Res Rev. 2016 Dec;32:13-21. doi: 10.1016/j.arr.2016.07.001. Epub 2016 Jul 30. Ageing Res Rev. 2016. PMID: 27484893 Review. - Chaperone-mediated autophagy.
Dice JF. Dice JF. Autophagy. 2007 Jul-Aug;3(4):295-9. doi: 10.4161/auto.4144. Epub 2007 Jul 15. Autophagy. 2007. PMID: 17404494 Review. - Chaperone-mediated autophagy.
Kaushik S, Cuervo AM. Kaushik S, et al. Methods Mol Biol. 2008;445:227-44. doi: 10.1007/978-1-59745-157-4_15. Methods Mol Biol. 2008. PMID: 18425454 Free PMC article. - Lysosome membrane lipid microdomains: novel regulators of chaperone-mediated autophagy.
Kaushik S, Massey AC, Cuervo AM. Kaushik S, et al. EMBO J. 2006 Sep 6;25(17):3921-33. doi: 10.1038/sj.emboj.7601283. Epub 2006 Aug 17. EMBO J. 2006. PMID: 16917501 Free PMC article.
Cited by
- Assessment of oxidative stress and activities of antioxidant enzymes depicts the negative systemic effect of iron-containing fertilizers and plant phenolic compounds in the desert locust.
Renault D, Dorrah MA, Mohamed AA, Abdelfattah EA, Bassal TT. Renault D, et al. Environ Sci Pollut Res Int. 2016 Nov;23(21):21989-22000. doi: 10.1007/s11356-016-7391-9. Epub 2016 Aug 18. Environ Sci Pollut Res Int. 2016. PMID: 27539469 - Chaperone-mediated autophagy regulates adipocyte differentiation.
Kaushik S, Juste YR, Lindenau K, Dong S, Macho-González A, Santiago-Fernández O, McCabe M, Singh R, Gavathiotis E, Cuervo AM. Kaushik S, et al. Sci Adv. 2022 Nov 18;8(46):eabq2733. doi: 10.1126/sciadv.abq2733. Epub 2022 Nov 16. Sci Adv. 2022. PMID: 36383673 Free PMC article. - Pros and Cons of Chaperone-Mediated Autophagy in Cancer Biology.
Arias E, Cuervo AM. Arias E, et al. Trends Endocrinol Metab. 2020 Jan;31(1):53-66. doi: 10.1016/j.tem.2019.09.007. Epub 2019 Nov 4. Trends Endocrinol Metab. 2020. PMID: 31699565 Free PMC article. Review. - Chaperone-mediated autophagy.
Bejarano E, Cuervo AM. Bejarano E, et al. Proc Am Thorac Soc. 2010 Feb;7(1):29-39. doi: 10.1513/pats.200909-102JS. Proc Am Thorac Soc. 2010. PMID: 20160146 Free PMC article. Review. - Cell Autophagy in NASH and NASH-Related Hepatocellular Carcinoma.
Udoh US, Rajan PK, Nakafuku Y, Finley R, Sanabria JR. Udoh US, et al. Int J Mol Sci. 2022 Jul 13;23(14):7734. doi: 10.3390/ijms23147734. Int J Mol Sci. 2022. PMID: 35887082 Free PMC article. Review.
References
- Agarraberes, F.A., and Dice, J.F. (2001). A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J. Cell Sci. 114, 2491-2499. - PubMed
- Aniento, F., Roche, E., Cuervo, A.M., and Knecht, E. (1993). Uptake and degradation of glyceraldehyde-3-phosphate dehydrogenase by rat liver lysosomes. J. Biol. Chem. 268, 10463-10470. - PubMed
- Brunk, U.T., Zhang, H., Dalen, H., and Ollinger, K. (1995). Exposure of cells to nonlethal concentrations of hydrogen peroxide induces degeneration-repair mechanisms involving lysosomal destabilization. Free Radic. Biol. Med. 19, 813-822. - PubMed
- Carr, A.C. (2001). Hypochlorous acid-modified low-density lipoprotein inactivates the lysosomal protease cathepsin B: protection by ascorbic and lipoic acids. Redox Rep. 6, 343-349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources