Dual effect of CD4+CD25+ regulatory T cells in neurodegeneration: a dialogue with microglia - PubMed (original) (raw)
Comparative Study
. 2004 Oct 5;101 Suppl 2(Suppl 2):14663-9.
doi: 10.1073/pnas.0404842101. Epub 2004 Aug 26.
Affiliations
- PMID: 15331781
- PMCID: PMC521988
- DOI: 10.1073/pnas.0404842101
Comparative Study
Dual effect of CD4+CD25+ regulatory T cells in neurodegeneration: a dialogue with microglia
Jonathan Kipnis et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Autoimmune CD4(+) T cells can mediate the ability to withstand neurodegenerative conditions. Here we show that the ability to spontaneously manifest a T cell-dependent protective response is restricted by naturally occurring CD4(+)CD25(+) regulatory T cells (Treg); depletion of Treg was beneficial in two mouse strains (C57BL/6J and BALB/c/OLA) differing in their spontaneous T cell-dependent ability to withstand the consequences of optic nerve injury. Passive transfer of exogenous Treg was destructive in BALB/c/OLA mice (which can spontaneously manifest a T cell-dependent protective anti-self response to injury) but beneficial in C57BL/6J mice (which have only limited ability to manifest such a response). This dichotomy was resolved by the finding that, in severe combined immunodeficient mice, a beneficial effect is obtained by passive transfer of either Treg-free CD4(+) T cells (Teff) or Treg alone, indicating that neuroprotection can be achieved by either Treg or Teff in the absence of the other. We attribute these disparate effects of Treg to their differential interaction (in part via IL-10 and transforming growth factor beta) with local innate immune cells (microglia) in the presence and in the absence of effector T cells. Activation of microglia by pro- and antiinflammatory cytokines in suitably controlled amounts might trigger different signal transduction pathways, each of which induces a neuroprotective microglial phenotype. These results suggest that, under neurodegenerative conditions, the effects of Treg, and possibly also of other regulatory T cells, might not be uniform, and that their expression in different individuals might be genetically determined. Therefore, therapeutic intervention based on induction of regulatory T cells might have limitations.
Figures
Fig. 1.
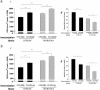
Neuroprotection induced in BALB/c/OLA and C57BL/6J mice by vaccination with retinal-specific antigens. BALB/c/OLA and C57BL/6J mice were immunized with 50 μg of IRBP (a) or S-Ag (b) or (as a control) with PBS, all emulsified in complete Freund's adjuvant. Significantly more neurons survived glutamate toxicity in PBS-immunized BALB/c/OLA mice than in PBS-immunized C57BL/6J mice. Immunization with each of the retinal antigens increased neuronal survival in both strains. Mean numbers of RGCs per mm2 are shown (ai and bi). Neuronal cell death is presented as a percentage of the mean total number of neurons in wild-type mice. (P values between groups, obtained by two-tailed Student's t test, are indicated by asterisks above the graph bars: **, P < 0.01; ***, P < 0.001; n = 6–8 in each group.)
Fig. 2.
Decreased neuronal loss in C57BL/6J mice after depletion or exogenous supply of naturally occurring regulatory CD4+CD25+ T cells. (a) Adult C57BL/6J mice that had been thymectomized 3 days after birth and wild-type adult mice of the same strain were injected intravitreally with a toxic dose of glutamate (400 nmol). Significantly more RGCs survived in the thymectomized mice than in control (nonthymectomized) age-matched mice (P < 0.001; Student's t test; n = 5–6 in each group, indicated by ***). (b) Adult C67BL/6J mice were injected with 1.5 × 106 CD4+CD25+ T cells (Treg) or with PBS (control) and were then exposed to intravitreal glutamate toxicity (400 nmol). In mice that received an exogenous supply of Treg, RGC loss was significantly decreased relative to PBS- or Teff-injected control mice (P < 0.05; two-tailed Student's t test; n = 7–8 in each group, indicated by *). For ease of comparison between the two sets of experiments, graph bars indicate the percentage of RGC loss.
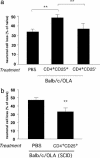
Fig. 3.
Differential effect of CD4+CD25+ regulatory T cells on neuronal survival in BALB/c/OLA and BALB/c/OLA SCID mice. (a) Naïve adult BALB/c/OLA mice injected with 1.5 × 106 CD4+CD25+ T cells (Treg) or 1.5 × 106 CD4+CD25- T cells (Teff) were exposed to intravitreal glutamate toxicity (400 nmol). Significantly fewer RGCs survived in mice that received Treg than in either PBS-treated control mice or mice treated with Teff (P < 0.01; two-tailed Student's t test; n = 8 in each group, indicated by **). (b) SCID mice of the BALB/c/OLA strain were injected with 1.5 × 106 CD4+CD25+ T cells (Treg) or with PBS (control) and were then exposed to intravitreal glutamate toxicity (400 nmol). Significantly more RGCs survived in the mice that had received Treg than in PBS-treated control mice (P < 0.01; two-tailed Student's t test; n = 6–7 in each group, indicated by **). For ease of comparison between the two sets of experiments, graph bars indicate percentage of RGC loss.
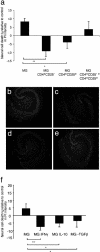
Fig. 4.
Decreased neuronal death in mouse organotypic hippocampal slices treated with activated microglia. OHSCs were obtained from BALB/c/OLA mice. Immediately after sectioning, the slices were cocultured for 24 h with microglia that had been preincubated (12 h) with CD4+CD25+ T cells (Treg) or CD4+CD25- T cells (Teff), or with a combination of Treg and Teff, or with microglia preincubated with IFN-γ, IL-10, or TGF-β. In all experiments, control slices were treated with naïve microglia or were left untreated. Twenty-four hours after coculturing of brain slices and microglia, slices were stained with PI (a fluorescent dye that stains only dead cells) and analyzed by fluorescence microscopy. (a) Intensity of PI in the treated groups, calculated as a percentage of the intensity measured in untreated control OHSCs (*, P < 0.05; **, P < 0.01; two-tailed Student's t test; n = 6–8 slices per group). (b) Representative micrograph of slices treated with naïve microglia. (c) Slices treated with microglia preincubated with Treg. (d) Slices treated with microglia preincubated with Teff. (e) Slices treated with microglia preincubated with a combination of Treg and Teff. (f) Intensity of PI in slices treated with the indicated cytokines, calculated as percentage of the intensity measured in untreated control OHSCs.
Similar articles
- Controlled autoimmunity in CNS maintenance and repair: naturally occurring CD4+CD25+ regulatory T-Cells at the crossroads of health and disease.
Kipnis J, Schwartz M. Kipnis J, et al. Neuromolecular Med. 2005;7(3):197-206. doi: 10.1385/NMM:7:3:197. Neuromolecular Med. 2005. PMID: 16247180 Review. - Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system.
Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Kipnis J, et al. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15620-5. doi: 10.1073/pnas.232565399. Epub 2002 Nov 12. Proc Natl Acad Sci U S A. 2002. PMID: 12429857 Free PMC article. - An instructive role of donor macrophages in mixed chimeras in the induction of recipient CD4(+)Foxp3(+) Treg cells.
Liu G, Duan K, Ma H, Niu Z, Peng J, Zhao Y. Liu G, et al. Immunol Cell Biol. 2011 Nov;89(8):827-35. doi: 10.1038/icb.2011.65. Epub 2011 Aug 16. Immunol Cell Biol. 2011. PMID: 21844881 - Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice.
Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, Zhao Y. Liu G, et al. Immunol Cell Biol. 2011 Jan;89(1):130-42. doi: 10.1038/icb.2010.70. Epub 2010 Jun 1. Immunol Cell Biol. 2011. PMID: 20514074 - CD4(+)CD25high regulatory T cells in human pregnancy.
Saito S, Sasaki Y, Sakai M. Saito S, et al. J Reprod Immunol. 2005 Apr;65(2):111-20. doi: 10.1016/j.jri.2005.01.004. J Reprod Immunol. 2005. PMID: 15811516 Review.
Cited by
- Alpha-Synuclein-Specific Regulatory T Cells Ameliorate Parkinson's Disease Progression in Mice.
Park SY, Yang H, Kim S, Yang J, Go H, Bae H. Park SY, et al. Int J Mol Sci. 2023 Oct 16;24(20):15237. doi: 10.3390/ijms242015237. Int J Mol Sci. 2023. PMID: 37894917 Free PMC article. - Imbalance of Circulating Th17 and Regulatory T Cells in Alzheimer's Disease: A Case Control Study.
Oberstein TJ, Taha L, Spitzer P, Hellstern J, Herrmann M, Kornhuber J, Maler JM. Oberstein TJ, et al. Front Immunol. 2018 Jun 4;9:1213. doi: 10.3389/fimmu.2018.01213. eCollection 2018. Front Immunol. 2018. PMID: 29915582 Free PMC article. - Lupus brain fog: a biologic perspective on cognitive impairment, depression, and fatigue in systemic lupus erythematosus.
Mackay M. Mackay M. Immunol Res. 2015 Dec;63(1-3):26-37. doi: 10.1007/s12026-015-8716-3. Immunol Res. 2015. PMID: 26481913 Review. - Controlled autoimmunity in CNS maintenance and repair: naturally occurring CD4+CD25+ regulatory T-Cells at the crossroads of health and disease.
Kipnis J, Schwartz M. Kipnis J, et al. Neuromolecular Med. 2005;7(3):197-206. doi: 10.1385/NMM:7:3:197. Neuromolecular Med. 2005. PMID: 16247180 Review. - Regulatory T cell in stroke: a new paradigm for immune regulation.
Chen S, Wu H, Klebe D, Hong Y, Zhang J, Tang J. Chen S, et al. Clin Dev Immunol. 2013;2013:689827. doi: 10.1155/2013/689827. Epub 2013 Aug 4. Clin Dev Immunol. 2013. PMID: 23983771 Free PMC article. Review.
References
- Kipnis, J., Yoles, E., Mizrahi, T., Ben-Nun, A. & Schwartz, M. (2002) J. Neuroimmunol. 130, 78-85. - PubMed
- Moalem, G., Leibowitz-Amit, R., Yoles, E., Mor, F., Cohen, I. R. & Schwartz, M. (1999) Nat. Med. 5, 49-55. - PubMed
- Moalem, G., Monsonego, A., Shani, Y., Cohen, I. R. & Schwartz, M. (1999) FASEB J. 13, 1207-1217. - PubMed
- Moalem, G., Gdalyahu, A., Shani, Y., Otten, U., Lazarovici, P., Cohen, I. R. & Schwartz, M. (2000) J. Autoimmun. 15, 331-345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials