The small chemical vacuolin-1 inhibits Ca(2+)-dependent lysosomal exocytosis but not cell resealing - PubMed (original) (raw)
The small chemical vacuolin-1 inhibits Ca(2+)-dependent lysosomal exocytosis but not cell resealing
Jan Cerny et al. EMBO Rep. 2004 Sep.
Erratum in
- EMBO Rep. 2005 Sep;6(9):898
Abstract
Resealing after wounding, the process of repair following plasma membrane damage, requires exocytosis. Vacuolins are molecules that induce rapid formation of large, swollen structures derived from endosomes and lysosomes by homotypic fusion combined with uncontrolled fusion of the inner and limiting membranes of these organelles. Vacuolin-1, the most potent compound, blocks the Ca(2+)-dependent exocytosis of lysosomes induced by ionomycin or plasma membrane wounding, without affecting the process of resealing. In contrast, other cell structures and membrane trafficking functions including exocytosis of enlargeosomes are unaffected. Because cells heal normally in the presence of vacuolin-1, we suggest that lysosomes are dispensable for resealing.
Figures
Figure 1
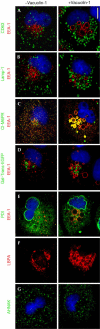
Origin of the vacuoles induced by vacuolin-1. BSC-1 cells were labelled with the indicated markers in the absence (left) or following 1 h incubation with 1 μM vacuolin-1 (right). Vacuolin-1 exhibits a vacuolating effect specific for endosomal and lysosomal compartments, manifested by generation of relatively large vacuoles (early endosomes) and small vacuoles (late endosomes and lysosomes). The identity of the resulting vacuoles is maintained, as the markers for each compartment do not intermix. In contrast, the enlargeosomes, Golgi apparatus, ER and nucleus (4,6-diamidino-2-phenylindole staining, in blue) are not affected, and the vacuoles do not contain markers from these organelles. Antibody markers are specific for early endosomes (EEA1), early and late endosomes (CI-M6PR), late endosomes (CD63 and LBPA), lysosomes (Lamp-1), the Golgi apparatus (Gal-Trans-EGFP), ER (PDI) and enlargeosomes (desmoyokin/AHNAK). Scale bar, 10 μM.
Figure 2
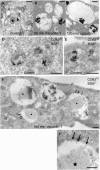
Enlargement of endosomes and lysosomes and loss of their inner membranes by treatment with vacuolin-1. HeLa cells were incubated for 10 min with bovine serum albumin (BSA) conjugated to 5 nm gold particles to mark endocytic compartments, followed by an overnight (A–C) or 3 h (D–F) chase. Control samples (A,D,E) were not incubated with vacuolin-1. The remaining samples were treated before fixation with 1 μM vacuolin-1 as follows: (B) 20 min, (C) 120 min and (F) 180 min. (A,D,E) Representative examples of late endosomal compartments in control cells showing BSA–gold within internal vesicles and membrane sheets of late endosomes/lysosomes primarily labelled with CD63. (B), (C) and (F) show a marked loss of inner membranes on treatment with vacuolin-1 and the frequent redistribution of the CD63 marker to the limiting membranes (arrows). The CD63-positive endosomes are hugely swollen (note the differences in bar sizes). Some endosomes contain few or no internal vesicles; instead, many have an amorphous content (asterisks) that is never observed in control cells. G, Golgi complex; P, plasma membrane. (F′) An enlarged portion of (F). Sections were stained with osmium (A–C) or ultrathin cryosections were prepared and labelled for CD63 (10 nm gold particles; D–F). Scale bars: (A–C, F′) 500 nm; (D–F) 250 nm.
Figure 3
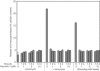
Vacuolin-1 blocks the Ca2+-dependent release of β-hexosaminidase from lysosomes. HeLa cells were incubated for the indicated times in the presence or absence of vacuolin-1 and then treated or not with 5 μM ionomycin for 10 min. Alternatively, the cells were wounded by gently rolling glass beads on top of the cell monolayer. The media were then exchanged, and the amount of β-hexosaminidase released during 10 min was determined. Data, obtained in quadruplicate, are expressed as average ± standard error normalized to the total cellular content of β-hexosaminidase. The same results were obtained with BSC-1 cells (not shown).
Figure 4
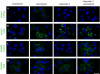
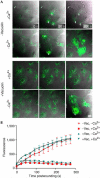
Vacuolin-1 blocks ionomycin-induced exocytosis of lysosomes but not of enlargeosomes. The fluorescent images show the effect of vacuolin-1 on the intracellular distribution in BSC-1 cells of the markers Lamp-1, specific for lysosomes (A), (B), and desmoyokin/AHNAK, specific for enlargeosomes (C), (D). The cells were immunostained before or after permeabilization with 0.05% saponin to identify the fusion of lysosomes and enlargeosomes with the plasma membrane and their intracellular location, respectively. In resting cells, lysosomes stain as cytosolic dots slightly larger than those corresponding to enlargeosomes. After treatment with ionomycin (5 μM, 5 min), both markers shift towards the cell periphery and appear at the surface, reflecting the Ca2+-regulated exocytosis of lysosomes (A), (B) and enlargeosomes (C), (D). Pretreatment with 1 μM vacuolin-1 results in vacuolation of lysosomes (A) and complete inhibition of the surface expression of Lamp-1 (D). In contrast, the appearance (C) and the exocytosis of the enlargeosome marker (D) remain normal. Vacuolin-1 alone does not induce surface expression of any marker. Scale bars, 20 μM.
Figure 5
Vacuolin does not inhibit laser-induced resealing. (A) BSC-1 cells exposed to DMSO (−vacuolin) or 10 μM vacuolin-1 for 1 h (+vacuolin) are shown before (0 s) and after (30 and 200 s) laser-mediated wounding. Burn marks in the plastic indicate the wound sites. Entry of the FM 1–43 dye present in the medium is restricted in cells wounded in the presence of 1.5 mM Ca2+ (+Ca2+), regardless of whether the cells were treated or not with vacuolin-1. In contrast, the FM 1–43 dye freely enters and stains intracellular membranes in cells wounded in the absence of Ca2+ (−Ca2+). (B) Quantitative evaluation of dye uptake in the absence (−Vac) or presence (+Vac) of vacuolin-1 before (_t_=0 s) and after laser wounding (for each data point, _n_=6 cells, scale bars=1 s.d.).
Similar articles
- The small chemical vacuolin-1 alters the morphology of lysosomes without inhibiting Ca2+-regulated exocytosis.
Huynh C, Andrews NW. Huynh C, et al. EMBO Rep. 2005 Sep;6(9):843-7. doi: 10.1038/sj.embor.7400495. EMBO Rep. 2005. PMID: 16113649 Free PMC article. - Vacuolin-1 potently and reversibly inhibits autophagosome-lysosome fusion by activating RAB5A.
Lu Y, Dong S, Hao B, Li C, Zhu K, Guo W, Wang Q, Cheung KH, Wong CW, Wu WT, Markus H, Yue J. Lu Y, et al. Autophagy. 2014;10(11):1895-905. doi: 10.4161/auto.32200. Epub 2014 Oct 30. Autophagy. 2014. PMID: 25483964 Free PMC article. - Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells.
Rodríguez A, Webster P, Ortego J, Andrews NW. Rodríguez A, et al. J Cell Biol. 1997 Apr 7;137(1):93-104. doi: 10.1083/jcb.137.1.93. J Cell Biol. 1997. PMID: 9105039 Free PMC article. - Lysosomes and plasma membrane repair.
Corrotte M, Castro-Gomes T. Corrotte M, et al. Curr Top Membr. 2019;84:1-16. doi: 10.1016/bs.ctm.2019.08.001. Epub 2019 Sep 3. Curr Top Membr. 2019. PMID: 31610859 Review. - Dealing with damage: plasma membrane repair mechanisms.
Draeger A, Schoenauer R, Atanassoff AP, Wolfmeier H, Babiychuk EB. Draeger A, et al. Biochimie. 2014 Dec;107 Pt A:66-72. doi: 10.1016/j.biochi.2014.08.008. Epub 2014 Aug 27. Biochimie. 2014. PMID: 25183513 Review.
Cited by
- Prion protein conversion at two distinct cellular sites precedes fibrillisation.
Ribes JM, Patel MP, Halim HA, Berretta A, Tooze SA, Klöhn PC. Ribes JM, et al. Nat Commun. 2023 Dec 15;14(1):8354. doi: 10.1038/s41467-023-43961-1. Nat Commun. 2023. PMID: 38102121 Free PMC article. - The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel.
Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. Dong XP, et al. Nature. 2008 Oct 16;455(7215):992-6. doi: 10.1038/nature07311. Epub 2008 Sep 14. Nature. 2008. PMID: 18794901 Free PMC article. - Patch-clamp technique to characterize ion channels in enlarged individual endolysosomes.
Chen CC, Cang C, Fenske S, Butz E, Chao YK, Biel M, Ren D, Wahl-Schott C, Grimm C. Chen CC, et al. Nat Protoc. 2017 Aug;12(8):1639-1658. doi: 10.1038/nprot.2017.036. Epub 2017 Jul 20. Nat Protoc. 2017. PMID: 28726848 - Enlargeosome, an exocytic vesicle resistant to nonionic detergents, undergoes endocytosis via a nonacidic route.
Cocucci E, Racchetti G, Podini P, Rupnik M, Meldolesi J. Cocucci E, et al. Mol Biol Cell. 2004 Dec;15(12):5356-68. doi: 10.1091/mbc.e04-07-0577. Epub 2004 Oct 6. Mol Biol Cell. 2004. PMID: 15469985 Free PMC article. - Dual role of HDAC10 in lysosomal exocytosis and DNA repair promotes neuroblastoma chemoresistance.
Ridinger J, Koeneke E, Kolbinger FR, Koerholz K, Mahboobi S, Hellweg L, Gunkel N, Miller AK, Peterziel H, Schmezer P, Hamacher-Brady A, Witt O, Oehme I. Ridinger J, et al. Sci Rep. 2018 Jul 3;8(1):10039. doi: 10.1038/s41598-018-28265-5. Sci Rep. 2018. PMID: 29968769 Free PMC article.
References
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP (2003) Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423: 168–172 - PubMed
- Borgonovo B, Cocucci E, Racchetti G, Podini P, Bachi A, Meldolesi J (2002) Regulated exocytosis: a novel, widely expressed system. Nat Cell Biol 4: 955–962 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous