Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation - PubMed (original) (raw)
Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation
M Lamar Seibenhener et al. Mol Cell Biol. 2004 Sep.
Abstract
Herein, we demonstrate that the ubiquitin-associated (UBA) domain of sequestosome 1/p62 displays a preference for binding K63-polyubiquitinated substrates. Furthermore, the UBA domain of p62 was necessary for aggregate sequestration and cell survival. However, the inhibition of proteasome function compromised survival in cells with aggregates. Mutational analysis of the UBA domain reveals that the conserved hydrophobic patch MGF as well as the conserved leucine in helix 2 are necessary for binding polyubiquitinated proteins and for sequestration-aggregate formation. We report that p62 interacts with the proteasome by pull-down assay, coimmunoprecipitation, and colocalization. Depletion of p62 levels results in an inhibition of ubiquitin proteasome-mediated degradation and an accumulation of ubiquitinated proteins. Altogether, our results support the hypothesis that p62 may act as a critical ubiquitin chain-targeting factor that shuttles substrates for proteasomal degradation.
Figures
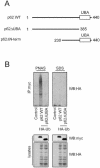
FIG. 1.
UBA domain binds polyubiquitin noncovalently and is required for aggregate formation. (A) The p62 constructs employed were myc-tagged wild-type (WT) p62; full-length p62 (amino acids 1 to 440); p62ΔN-term, a construct missing amino acids 1 to 229; and p62ΔUBA, a construct missing the UBA domain (amino acids 386 to 440). (B) myc-tagged full-length p62 construct or _myc_-tagged construct lacking the UBA domain (p62ΔUBA) were transfected into HEK cells along with HA-tagged ubiquitin (HA-Ub). Lysis and immunoprecipitation (IP) were carried out in either PNAS buffer (lacking SDS) or SDS lysis buffer (SDS) followed by SDS-PAGE and Western blotting (WB) for HA-tagged ubiquitin. (C) Shown are laser scanning confocal microscopy images of immunofluorescence staining for exogenous myc-p62 (red) and HA-ubiquitin (green) of wild-type myc-p62-expressing (top), myc-p62ΔUBA-expressing (middle), and myc-p62ΔN-term-expressing (bottom) HEK cells with (+) or without (−) proteasomal inhibitor ALLN (50 μM) for 24 h. Cells were incubated with rabbit anti-myc IgG or mouse anti-HA IgG and labeled with Texas Red-conjugated anti-rabbit antibodies (red) or Oregon Green-conjugated anti-mouse antibodies (green), respectively. Merged images with overlapping immunoreactivity are shown in yellow. Note that cells expressing p62 lacking its UBA domain (p62ΔUBA) fail to form aggregates or to colocalize with ubiquitin. All experiments were replicated three independent times with similar results. (D) HEK cells (in a 24-well plate) were transfected with myc-tagged wild-type p62, p62ΔUBA, or p62ΔN-term along with HA-tagged ubiquitin constructs. After 24 h of transfection, the cells were treated with or without ALLN (50 μM) for 30 h. Cell survival was assessed by the addition of MTS reagent for 2 h. Values shown are means ± standard error of the means of four different experiments. Survival was significantly diminished between the control and p62ΔUBA (P < 0.001) and between wild-type p62 and p62ΔN-term (P < 0.001).
FIG. 1.
UBA domain binds polyubiquitin noncovalently and is required for aggregate formation. (A) The p62 constructs employed were myc-tagged wild-type (WT) p62; full-length p62 (amino acids 1 to 440); p62ΔN-term, a construct missing amino acids 1 to 229; and p62ΔUBA, a construct missing the UBA domain (amino acids 386 to 440). (B) myc-tagged full-length p62 construct or _myc_-tagged construct lacking the UBA domain (p62ΔUBA) were transfected into HEK cells along with HA-tagged ubiquitin (HA-Ub). Lysis and immunoprecipitation (IP) were carried out in either PNAS buffer (lacking SDS) or SDS lysis buffer (SDS) followed by SDS-PAGE and Western blotting (WB) for HA-tagged ubiquitin. (C) Shown are laser scanning confocal microscopy images of immunofluorescence staining for exogenous myc-p62 (red) and HA-ubiquitin (green) of wild-type myc-p62-expressing (top), myc-p62ΔUBA-expressing (middle), and myc-p62ΔN-term-expressing (bottom) HEK cells with (+) or without (−) proteasomal inhibitor ALLN (50 μM) for 24 h. Cells were incubated with rabbit anti-myc IgG or mouse anti-HA IgG and labeled with Texas Red-conjugated anti-rabbit antibodies (red) or Oregon Green-conjugated anti-mouse antibodies (green), respectively. Merged images with overlapping immunoreactivity are shown in yellow. Note that cells expressing p62 lacking its UBA domain (p62ΔUBA) fail to form aggregates or to colocalize with ubiquitin. All experiments were replicated three independent times with similar results. (D) HEK cells (in a 24-well plate) were transfected with myc-tagged wild-type p62, p62ΔUBA, or p62ΔN-term along with HA-tagged ubiquitin constructs. After 24 h of transfection, the cells were treated with or without ALLN (50 μM) for 30 h. Cell survival was assessed by the addition of MTS reagent for 2 h. Values shown are means ± standard error of the means of four different experiments. Survival was significantly diminished between the control and p62ΔUBA (P < 0.001) and between wild-type p62 and p62ΔN-term (P < 0.001).
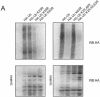
FIG. 2.
UBA domain binds K63-linked polyubiquitinated substrates. (A) HEK cells were transfected either with HA-tagged wild-type ubiquitin (HA-Ub) or K29R, K48R, or K63R point mutants of ubiquitin (left) or with HA-tagged wild-type ubiquitin or K63R, K29,48R, or K29,48,63R mutants of ubiquitin (right). An equal protein concentration of cell lysate was interacted with the UBA domain of p62 in a pull-down assay. Binding of polyubiquitin chains was determined by Western blot analysis with HA antibody to detect ubiquitin (WB:HA). The expression of ubiquitin constructs was verified by blotting an aliquot of the transfected cell lysate (40 μg) with HA tag antibody. (B) HEK cells were transfected with GFP-p62 followed by treatment with (+) or without (−) ALLN for 18 h prior to fixation. The cells were stained with antibody to TRAF6 with Texas Red as the secondary antibody. The colocalization of TRAF6 and p62 was determined by confocal microscopy. (C) HEK cells were pretreated with increasing doses (0, 50, 75, 150, and 300 μM) of control or TRAF6 inhibitory peptide for 5 h followed by treatment with IL-1 (10 ng/ml) for 15 min. Lysates were prepared (200 μg) and included in a GST-p62 UBA pull-down followed by SDS-PAGE and immunoblotting with antiubiquitin antibody. In parallel, the lysates (750 μg) were immunoprecipitated (IP) with TRAF6 antibody and blotted (WB) for p62. Lysates (40 μg) were blotted with both p62 and TRAF6 antibodies as shown. (D) HEK cells were transfected with GFP-p62 followed by treatment with TRAF6 inhibitory peptide (150 μM) for 2 h, followed by the addition of ALLN for an additional 12 h prior to fixation and visualization by confocal microscopy. All experiments were replicated three independent times with similar results.
FIG. 2.
UBA domain binds K63-linked polyubiquitinated substrates. (A) HEK cells were transfected either with HA-tagged wild-type ubiquitin (HA-Ub) or K29R, K48R, or K63R point mutants of ubiquitin (left) or with HA-tagged wild-type ubiquitin or K63R, K29,48R, or K29,48,63R mutants of ubiquitin (right). An equal protein concentration of cell lysate was interacted with the UBA domain of p62 in a pull-down assay. Binding of polyubiquitin chains was determined by Western blot analysis with HA antibody to detect ubiquitin (WB:HA). The expression of ubiquitin constructs was verified by blotting an aliquot of the transfected cell lysate (40 μg) with HA tag antibody. (B) HEK cells were transfected with GFP-p62 followed by treatment with (+) or without (−) ALLN for 18 h prior to fixation. The cells were stained with antibody to TRAF6 with Texas Red as the secondary antibody. The colocalization of TRAF6 and p62 was determined by confocal microscopy. (C) HEK cells were pretreated with increasing doses (0, 50, 75, 150, and 300 μM) of control or TRAF6 inhibitory peptide for 5 h followed by treatment with IL-1 (10 ng/ml) for 15 min. Lysates were prepared (200 μg) and included in a GST-p62 UBA pull-down followed by SDS-PAGE and immunoblotting with antiubiquitin antibody. In parallel, the lysates (750 μg) were immunoprecipitated (IP) with TRAF6 antibody and blotted (WB) for p62. Lysates (40 μg) were blotted with both p62 and TRAF6 antibodies as shown. (D) HEK cells were transfected with GFP-p62 followed by treatment with TRAF6 inhibitory peptide (150 μM) for 2 h, followed by the addition of ALLN for an additional 12 h prior to fixation and visualization by confocal microscopy. All experiments were replicated three independent times with similar results.
FIG. 2.
UBA domain binds K63-linked polyubiquitinated substrates. (A) HEK cells were transfected either with HA-tagged wild-type ubiquitin (HA-Ub) or K29R, K48R, or K63R point mutants of ubiquitin (left) or with HA-tagged wild-type ubiquitin or K63R, K29,48R, or K29,48,63R mutants of ubiquitin (right). An equal protein concentration of cell lysate was interacted with the UBA domain of p62 in a pull-down assay. Binding of polyubiquitin chains was determined by Western blot analysis with HA antibody to detect ubiquitin (WB:HA). The expression of ubiquitin constructs was verified by blotting an aliquot of the transfected cell lysate (40 μg) with HA tag antibody. (B) HEK cells were transfected with GFP-p62 followed by treatment with (+) or without (−) ALLN for 18 h prior to fixation. The cells were stained with antibody to TRAF6 with Texas Red as the secondary antibody. The colocalization of TRAF6 and p62 was determined by confocal microscopy. (C) HEK cells were pretreated with increasing doses (0, 50, 75, 150, and 300 μM) of control or TRAF6 inhibitory peptide for 5 h followed by treatment with IL-1 (10 ng/ml) for 15 min. Lysates were prepared (200 μg) and included in a GST-p62 UBA pull-down followed by SDS-PAGE and immunoblotting with antiubiquitin antibody. In parallel, the lysates (750 μg) were immunoprecipitated (IP) with TRAF6 antibody and blotted (WB) for p62. Lysates (40 μg) were blotted with both p62 and TRAF6 antibodies as shown. (D) HEK cells were transfected with GFP-p62 followed by treatment with TRAF6 inhibitory peptide (150 μM) for 2 h, followed by the addition of ALLN for an additional 12 h prior to fixation and visualization by confocal microscopy. All experiments were replicated three independent times with similar results.
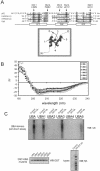
FIG. 3.
Critical determinants of p62-polyubiquitin interaction. (A) Sequence alignment of the UBA domain of p62 with UBA containing proteins hHR23A UBA1, hHR23A UBA2, and Ddi1, indicating conserved residues and α-helix formation. Arrows indicate residues mutated for analysis as well as their location on a three-dimensional model of the p62 UBA domain. (B) Overlaid circular dichroism spectra of UBA peptides. (C) HEK cells were transfected with HA-tagged wild-type ubiquitin (WT-Ub) or left untransfected as the control. Pull-down assays were carried out by using the GST-UBA domain of p62 and its mutants UBA1, UBA2, UBA3, UBA4, and UBA5. The lysates (500 μg) were interacted with 5 μg of GST-UBA in a pull-down assay. The interaction of polyubiquitin chains with each UBA construct was analyzed by immunoblotting (WB) with antibody to ubiquitin. The findings were replicated three independent times with similar results. To validate equal amounts of GST-UBA in the pull-down assay, the blot (∼32kDa) was probed with GST antibody. The expression of HA-tagged ubiquitin was verified by immunoblotting with HA antibody.
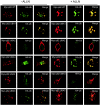
FIG. 4.
UBA-polyubiquitin interaction is required for aggregate formation. HEK cells were cotransfected with HA-tagged ubiquitin (HA-Ub) together with myc-tagged full-length and UBA mutants of p62 expression constructs treated with (+) or without (−) proteasomal inhibitor ALLN (50 μM) for 24 h and then labeled with antibodies to HA and myc. Images of a representative cell are shown. Anti-myc labeling (red) detects wild-type (WT) myc-p62 and its UBA domain mutants. Anti-HA labeling (green) detects ubiquitin. Merged images show the overlap between the individual staining patterns as yellow. The findings are representative of two independent experiments.
FIG. 5.
p62 interacts with the proteasome. (A) myc-tagged constructs wild-type (WT) p62, p62ΔUBA, and p62ΔN-term were expressed in HEK cells, and lysates were prepared as described in Materials and Methods. An equal amount of GST-S5a agarose was added to 500 μg of lysate from each sample and incubated for 2 h at 30°C. Following interaction, beads were washed, and myc-tagged constructs bound to S5a were analyzed by SDS-PAGE and Western blotting (WB) with myc antibody. Construct expression and the amount of GST-S5a input into the assay were also analyzed by Western blot with the appropriate antibody as shown. All experiments were replicated three independent times with similar results. (B) p62 interacts with proteasomes in a stimulus-dependent manner. HEK cells were treated with IL-1 (10 ng/ml) for 5 min followed by immunoprecipitation (IP) with p62 antibody and immunoblotting with Rpt1 (left). Alternatively, myc-tagged p62 constructs were expressed in HEK cells followed by stimulation with IL-1 (10 ng/ml). The lysates (750 μg) were immunoprecipitated with Rpt1 antibody and immunoblotted with myc to detect the tagged p62 constructs (right; shown with an asterisk). As the control, the expression of the proteins in the lysate (40 μg) was determined. (C) p62 interacts directly with the proteasome. Immobilized bacterially expressed GST-p62 (5 μg) or GST control was incubated with purified 26S proteasomes (0 to 1 μg). After washing, bound subunit Rpt1 was detected by immunoblotting. (D) p62 aggregates contain proteasomal subunits. HEK cells were cotransfected with GFP-p62 and treated with ALLN (50 μM) for 18 h and then labeled with antibodies to Rpn10/S5a, Rpt1, or proteasomal subunits as indicated. Images of a representative cell are shown. Green detects the GFP-p62 and red detects the endogenous proteins. Merged images show the overlap between the individual staining patterns in yellow.
FIG. 5.
p62 interacts with the proteasome. (A) myc-tagged constructs wild-type (WT) p62, p62ΔUBA, and p62ΔN-term were expressed in HEK cells, and lysates were prepared as described in Materials and Methods. An equal amount of GST-S5a agarose was added to 500 μg of lysate from each sample and incubated for 2 h at 30°C. Following interaction, beads were washed, and myc-tagged constructs bound to S5a were analyzed by SDS-PAGE and Western blotting (WB) with myc antibody. Construct expression and the amount of GST-S5a input into the assay were also analyzed by Western blot with the appropriate antibody as shown. All experiments were replicated three independent times with similar results. (B) p62 interacts with proteasomes in a stimulus-dependent manner. HEK cells were treated with IL-1 (10 ng/ml) for 5 min followed by immunoprecipitation (IP) with p62 antibody and immunoblotting with Rpt1 (left). Alternatively, myc-tagged p62 constructs were expressed in HEK cells followed by stimulation with IL-1 (10 ng/ml). The lysates (750 μg) were immunoprecipitated with Rpt1 antibody and immunoblotted with myc to detect the tagged p62 constructs (right; shown with an asterisk). As the control, the expression of the proteins in the lysate (40 μg) was determined. (C) p62 interacts directly with the proteasome. Immobilized bacterially expressed GST-p62 (5 μg) or GST control was incubated with purified 26S proteasomes (0 to 1 μg). After washing, bound subunit Rpt1 was detected by immunoblotting. (D) p62 aggregates contain proteasomal subunits. HEK cells were cotransfected with GFP-p62 and treated with ALLN (50 μM) for 18 h and then labeled with antibodies to Rpn10/S5a, Rpt1, or proteasomal subunits as indicated. Images of a representative cell are shown. Green detects the GFP-p62 and red detects the endogenous proteins. Merged images show the overlap between the individual staining patterns in yellow.
FIG. 6.
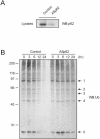
p62 is involved in ubiquitin proteasome degradation of polyubiquitinated proteins. (A) HEK cells were transfected with full-length antisense p62 (ASp62) construct or not. The levels of endogenous p62 were examined by immunoblotting lysates (40 μg) with p62 antibody. (B) Control or antisense p62-transfected HEK cells were treated with 20 mg of cycloheximide/ml as indicated. Whole-cell lysates (40 μg) were separated by SDS-PAGE and immunoblotted (WB) with antiubiquitin antibody. (C) The relative expression of five prominent polyubiquitinated proteins present in both control and antisense p62 lysates was determined and plotted as shown. The experiment was repeated twice with similar results.
FIG. 6.
p62 is involved in ubiquitin proteasome degradation of polyubiquitinated proteins. (A) HEK cells were transfected with full-length antisense p62 (ASp62) construct or not. The levels of endogenous p62 were examined by immunoblotting lysates (40 μg) with p62 antibody. (B) Control or antisense p62-transfected HEK cells were treated with 20 mg of cycloheximide/ml as indicated. Whole-cell lysates (40 μg) were separated by SDS-PAGE and immunoblotted (WB) with antiubiquitin antibody. (C) The relative expression of five prominent polyubiquitinated proteins present in both control and antisense p62 lysates was determined and plotted as shown. The experiment was repeated twice with similar results.
Similar articles
- Sequestosome 1/p62 shuttles polyubiquitinated tau for proteasomal degradation.
Babu JR, Geetha T, Wooten MW. Babu JR, et al. J Neurochem. 2005 Jul;94(1):192-203. doi: 10.1111/j.1471-4159.2005.03181.x. J Neurochem. 2005. PMID: 15953362 - Ubiquitin-mediated sequestration of normal cellular proteins into polyglutamine aggregates.
Donaldson KM, Li W, Ching KA, Batalov S, Tsai CC, Joazeiro CA. Donaldson KM, et al. Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):8892-7. doi: 10.1073/pnas.1530212100. Epub 2003 Jul 11. Proc Natl Acad Sci U S A. 2003. PMID: 12857950 Free PMC article. - Ubiquitin binding modulates IAP antagonist-stimulated proteasomal degradation of c-IAP1 and c-IAP2(1).
Blankenship JW, Varfolomeev E, Goncharov T, Fedorova AV, Kirkpatrick DS, Izrael-Tomasevic A, Phu L, Arnott D, Aghajan M, Zobel K, Bazan JF, Fairbrother WJ, Deshayes K, Vucic D. Blankenship JW, et al. Biochem J. 2009 Jan 1;417(1):149-60. doi: 10.1042/BJ20081885. Biochem J. 2009. PMID: 18939944 - Structural and functional studies of mutations affecting the UBA domain of SQSTM1 (p62) which cause Paget's disease of bone.
Layfield R, Ciani B, Ralston SH, Hocking LJ, Sheppard PW, Searle MS, Cavey JR. Layfield R, et al. Biochem Soc Trans. 2004 Nov;32(Pt 5):728-30. doi: 10.1042/BST0320728. Biochem Soc Trans. 2004. PMID: 15493999 Review. - Sequestosome 1/p62--more than just a scaffold.
Seibenhener ML, Geetha T, Wooten MW. Seibenhener ML, et al. FEBS Lett. 2007 Jan 23;581(2):175-9. doi: 10.1016/j.febslet.2006.12.027. Epub 2006 Dec 19. FEBS Lett. 2007. PMID: 17188686 Free PMC article. Review.
Cited by
- Molecular mechanisms regulating formation, trafficking and processing of annular gap junctions.
Falk MM, Bell CL, Kells Andrews RM, Murray SA. Falk MM, et al. BMC Cell Biol. 2016 May 24;17 Suppl 1(Suppl 1):22. doi: 10.1186/s12860-016-0087-7. BMC Cell Biol. 2016. PMID: 27230503 Free PMC article. Review. - RBM45 Modulates the Antioxidant Response in Amyotrophic Lateral Sclerosis through Interactions with KEAP1.
Bakkar N, Kousari A, Kovalik T, Li Y, Bowser R. Bakkar N, et al. Mol Cell Biol. 2015 Jul;35(14):2385-99. doi: 10.1128/MCB.00087-15. Epub 2015 May 4. Mol Cell Biol. 2015. PMID: 25939382 Free PMC article. - Autophagy and ALS: mechanistic insights and therapeutic implications.
Chua JP, De Calbiac H, Kabashi E, Barmada SJ. Chua JP, et al. Autophagy. 2022 Feb;18(2):254-282. doi: 10.1080/15548627.2021.1926656. Epub 2021 May 31. Autophagy. 2022. PMID: 34057020 Free PMC article. Review. - Autophagy regulates tumor growth and metastasis.
Qiang L, Zhao B, Ming M, Wang N, He TC, Hwang S, Thorburn A, He YY. Qiang L, et al. bioRxiv [Preprint]. 2023 Nov 3:2023.10.31.564991. doi: 10.1101/2023.10.31.564991. bioRxiv. 2023. PMID: 37961427 Free PMC article. Preprint. - Rapamycin protects against gentamicin-induced acute kidney injury via autophagy in mini-pig models.
Cui J, Bai XY, Sun X, Cai G, Hong Q, Ding R, Chen X. Cui J, et al. Sci Rep. 2015 Jun 8;5:11256. doi: 10.1038/srep11256. Sci Rep. 2015. PMID: 26052900 Free PMC article.
References
- Alves-Rodrigues, A., L. Gregori, and M. E. Figueiredo-Pereira. 1998. Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends Neurosci. 21:516-520. - PubMed
- Bence, N. F., R. M. Sampat, and R. R. Kopito. 2001. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292:1552-1555. - PubMed
- Bertolaet, B. L., D. J. Clarke, M. Wolff, M. H. Watson, M. Henze, G. Divita, and S. I. Reed. 2001. UBA domains of DNA damage-inducible proteins interact with ubiquitin. Nat. Struct. Biol. 8:417-422. - PubMed
- Butterfield, S. M., P. R. Patel, and M. L. Waters. 2002. Contribution of aromatic interactions to α-helix stability. J. Am. Chem. Soc. 124:9751-9755. - PubMed
- Ciani, B., R. Layfield, J. R. Cavey, P. W. Sheppard, and M. S. Searle. 2003. Structure of the ubiquitin-associated domain of p62 (SQSTM1) and implications for mutations that cause Paget's disease of bone. J. Biol. Chem. 278:37409-37412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases