GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone - PubMed (original) (raw)
Comparative Study
GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone
Anna J Bolteus et al. J Neurosci. 2004.
Abstract
In the postnatal subventricular zone (SVZ), astrocyte-like cells tightly encapsulate chains of migrating neuronal precursors, although an influence of the astrocyte-like cells on precursor migration has not yet been demonstrated. Cell migration was studied in acute sagittal brain slices to determine whether GABA signaling between astrocyte-like cells and neuronal precursors controls the speed of neuronal precursor migration in the anterior SVZ and rostral migratory stream of juvenile and adult mice. Application of GABA at 10 microm, a nondesensitizing concentration for GABA(A) receptors (GABA(A)Rs), reduced the rate (mean of approximately 50 microm/hr) of cell migration by 21% via GABA(A)R activation. Application of the GABA(A)R antagonist bicuculline enhanced the migration rate by 30%, suggesting that endogenous GABA tonically reduces the speed of cell migration via GABA(A)R activation. Using immunohistochemistry, we found that astrocyte-like cells express the high-affinity GABA transporter subtype GAT4 on processes ensheathing neuronal precursors that contain GABA. Inhibition of GABA uptake into astrocyte-like cells or enhancement of GABA release from neuronal precursors during high K(+) application further reduced the migration rate by increasing ambient GABA levels. GABA altered the migration speed by interfering with intracellular Ca(2+) signaling independently of cell depolarization, because high K(+) application did not alter the speed of cell migration in the presence of bicuculline. These data indicate that astrocyte-like cells create a microenvironment in which their uniquely positioned GABA transporters control the degree of GABA(A)R activation and the migration of neuronal precursors.
Figures
Figure 1.
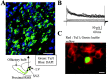
Identification of migrating cells as neuronal precursors. A, TuJ1 immunostaining in the anterior SVZ and early RMS of a sagittal slice. A schematic diagram under the photograph illustrates where the photograph was taken. Scale bar, 30 μm. LV, Lateral ventricle. B, C, Characteristic outwardly rectifying current traces in response to 20 mV depolarizing pulses from -100 to +80 mV (B) obtained in the Lucifer yellow-filled cell that stains positive for TuJ1 (C). Recorded cells were held at a holding potential of -70 mV. Scale bar, 10 μm. Staining and recordings were performed in slices from P14-P20 mice.
Figure 2.
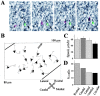
Time-lapse videomicroscopy of neuronal precursors migrating in the SVZ. A, Photographs of migrating cells in the anterior SVZ of an acute sagittal slice from a P17 mouse. A stationary cell is labeled in green and a cell migrating toward the olfactory bulb is labeled in red. Displayed images were taken every 5 min. Scale bar, 50 pixels corresponding to 8 μm. B, Schematic drawing illustrating the migratory routes of several precursors (arrowheads) in an acute sagittal slice. C, Average speed (in micrometers/hour) of cell migration (n = 45 cells from three slices from juvenile mice). D, Percentage of cells migrating in the different directions illustrated on the drawing in B (same cells as in C).
Figure 3.
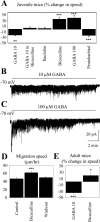
Ambient GABA reduces the speed of neuronal precursor migration via GABAAR activation. A, Average percentage change in the speed of neuronal precursor migration induced by applications of various drugs: 10 μ
m
GABA, GABA in the presence of 100 μ
m
bicuculline, 10 μ
m
baclofen (a GABABR agonist), 100 μ
m
bicuculline, 100 μ
m
GABA, or 50 μ
m
pentobarbital (a GABAAR allosteric enhancer) in juvenile mice. B, C, Inward GABAA currents in neuronal precursors induced by pressure applications of 10 (B) and 100 μ
m
(C) GABA (as indicated by the bar above the trace). Cells were recorded at a holding potential of -70 mV in acute slices from juvenile mice. D, Average speed (in micrometers/hour) of cell migration in slices from juvenile mice under control, during bicuculline application, and after washout of bicuculline. E, Average percentage change in the speed of neuronal precursor migration induced by 10 μ
m
GABA and by 100 μ
m
bicuculline on the speed of neuronal precursor migration rate in adult mice (3-4 months of age). The migration speeds of 90 cells (45 cells in control and 45 cells with a drug) from three slices were routinely measured per condition. **p < 0.01; ***p < 0.001.
Figure 4.
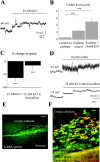
Depolarization-induced GABA release reduces the speed of cell migration via GABAAR activation. A, Depolarization induced by a pressure application of 25 m
m
K+ in neuronal precursors recorded in the current-clamp configuration. B, Mean concentration of GABA in the BME solution and in the supernatant (20 μl) of four explants under control and during 25 m
m
K+ application for 1 hr. C, Average percentage change in the speed of neuronal precursor migration induced by 25 m
m
K+ applications with and without 100 μ
m
bicuculline. The migration speeds of 90 cells (45 cells in control and 45 cells with a drug) from three slices were routinely measured per condition. D, Depolarizations induced by pressure application of 25 m
m
K+ in the presence of bicuculline or 10 μ
m
GABA in neuronal precursors recorded in the current-clamp configuration. Neuronal precursors were recorded with an intracellular solution containing 29 m
m
Cl-. E, GABA immunostaining (green) within the SVZ and RMS in a sagittal slice. The rectangle indicates the location of the photograph displayed in F. Scale bar, 100 μm. F, GABA (green) and TuJ1 (red) immunostainings in a sagittal slice. Immunostaining was performed in a different slice than that used for the staining shown in E. Scale bar, 10 μm. Experiments were performed in acute slices and RMS explants from P14-P20 mice. ***p < 0.001.
Figure 5.
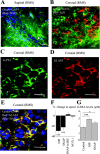
GABA uptake into astrocyte-like cells controls the speed of neuronal progenitor migration. A, Coimmunostaining for GAT4 (green; rat GAT-3) and DAPI (blue) in the anterior SVZ of a sagittal slice. Note the chains of precursors ensheathed by GAT4+ cells (arrows). Scale bar, 50 μm. B, Coimmunostaining for GAT4 (green) and TuJ1 (red) in the anterior SVZ in a coronal slice. C, D, Immunostainings for GAT4 (C, green) and GLAST (D, red) in the RMS of a coronal slice. E, Superimposition of the immunostainings for GAT4 (green), GLAST (red), and DAPI (blue) from slices shown in C and D. Scale bars: _B_-E, 20 μm. F, Average percentage decrease in the speed of neuronal precursor migration induced by 100 μ
m
nipecotic acid (NIP) or 50 μ
m
SNAP5114 (SNAP). BIC, Bicuculline. The migration speeds of 90 cells (45 cells in control and 45 cells with a drug) from three slices were routinely measured per condition. G, Mean concentration of GABA in the supernatant (20 μl) of four explants under control, in the presence of 100 μ
m
nipecotic acid, or 50 μ
m
SNAP5114. Experiments were performed in acute slices and explants from P14-P20 mice. *p < 0.01; ***p < 0.001.
Figure 6.
GABAAR activation reduces the speed of neuronal precursor migration by interfering with Ca2+ release from intracellular Ca2+ stores. A, Average percentage decrease in the speed of neuronal precursor migration induced by 1 m
m
EGTA, 10 μ
m
BAPTA-AM, or 100 μ
m
2-APB. ***p < 0.001. B, Average percentage change in the speed of neuronal precursor migration induced by 10 μ
m
GABA or bicuculline (BIC) in the presence of 100 μ
m
2-APB. The control speed of migration was measured in the presence of 2-APB. The migration speeds of 90 cells (45 cells in control and 45 cells with a drug) in three slices from juvenile mice were routinely measured per condition. *p < 0.05.
Figure 7.
Diagram illustrating the influence of GABA release from neuronal precursors and GABA uptake into astrocyte-like cells on neuronal precursor migration in the SVZ. GABA released from neuronal precursors activates GABAARs on surrounding neuronal precursors and reduces the speed of neuronal precursor migration by interacting with Ca2+ release from intracellular Ca2+ stores. Released GABA is taken up by surrounding astrocyte-like cells that ensheath neuronal precursors and create a microenvironment in which the levels of GABA and the degree of GABAAR activation are tightly controlled.
Similar articles
- Assays for measuring extracellular GABA levels and cell migration rate in acute slices.
Bolteus AJ, Garganta C, Bordey A. Bolteus AJ, et al. Brain Res Brain Res Protoc. 2005 Feb;14(2):126-34. doi: 10.1016/j.brainresprot.2004.12.005. Brain Res Brain Res Protoc. 2005. PMID: 15721818 - GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation.
Wang DD, Krueger DD, Bordey A. Wang DD, et al. J Physiol. 2003 Aug 1;550(Pt 3):785-800. doi: 10.1113/jphysiol.2003.042572. Epub 2003 Jun 13. J Physiol. 2003. PMID: 12807990 Free PMC article. - Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling.
Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Götz M, Barker PA, Parent A, Saghatelyan A. Snapyan M, et al. J Neurosci. 2009 Apr 1;29(13):4172-88. doi: 10.1523/JNEUROSCI.4956-08.2009. J Neurosci. 2009. PMID: 19339612 Free PMC article. - GABA effects during neuronal differentiation of stem cells.
Salazar P, Velasco-Velázquez MA, Velasco I. Salazar P, et al. Neurochem Res. 2008 Aug;33(8):1546-57. doi: 10.1007/s11064-008-9642-8. Epub 2008 Mar 21. Neurochem Res. 2008. PMID: 18357524 Review. - Role of tonic GABAergic currents during pre- and early postnatal rodent development.
Kilb W, Kirischuk S, Luhmann HJ. Kilb W, et al. Front Neural Circuits. 2013 Sep 3;7:139. doi: 10.3389/fncir.2013.00139. eCollection 2013. Front Neural Circuits. 2013. PMID: 24027498 Free PMC article. Review.
Cited by
- Neural stem cell niches in health and diseases.
Decimo I, Bifari F, Krampera M, Fumagalli G. Decimo I, et al. Curr Pharm Des. 2012;18(13):1755-83. doi: 10.2174/138161212799859611. Curr Pharm Des. 2012. PMID: 22394166 Free PMC article. Review. - GABAergic excitation after febrile seizures induces ectopic granule cells and adult epilepsy.
Koyama R, Tao K, Sasaki T, Ichikawa J, Miyamoto D, Muramatsu R, Matsuki N, Ikegaya Y. Koyama R, et al. Nat Med. 2012 Aug;18(8):1271-8. doi: 10.1038/nm.2850. Epub 2012 Jul 15. Nat Med. 2012. PMID: 22797810 - Cellular and molecular determinants of stroke-induced changes in subventricular zone cell migration.
Young CC, Brooks KJ, Buchan AM, Szele FG. Young CC, et al. Antioxid Redox Signal. 2011 May 15;14(10):1877-88. doi: 10.1089/ars.2010.3435. Epub 2010 Nov 1. Antioxid Redox Signal. 2011. PMID: 20673127 Free PMC article. Review. - Cell Signaling in Neuronal Stem Cells.
Navarro Quiroz E, Navarro Quiroz R, Ahmad M, Gomez Escorcia L, Villarreal JL, Fernandez Ponce C, Aroca Martinez G. Navarro Quiroz E, et al. Cells. 2018 Jul 14;7(7):75. doi: 10.3390/cells7070075. Cells. 2018. PMID: 30011912 Free PMC article. Review. - Dynamic imaging reveals that brain-derived neurotrophic factor can independently regulate motility and direction of neuroblasts within the rostral migratory stream.
Bagley JA, Belluscio L. Bagley JA, et al. Neuroscience. 2010 Sep 1;169(3):1449-61. doi: 10.1016/j.neuroscience.2010.05.075. Epub 2010 Jun 9. Neuroscience. 2010. PMID: 20538046 Free PMC article.
References
- Barker JL, Behar T, Li YX, Liu QY, Ma W, Maric D, Maric I, Schaffner AE, Serafini R, Smith SV, Somogyi R, Vautrin JY, Wen XL, Xian H (1998) GABAergic cells and signals in CNS development. Perspect Dev Neurobiol 5: 305-322. - PubMed
- Borden LA (1996) GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int 29: 335-356. - PubMed
- Boulder Committee (1970) Embryonic vertebrate central nervous system: revised terminology. Anat Rec 166: 257-261. - PubMed
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM (2003) Becoming a new neuron in the adult olfactory bulb. Nat Neurosci 6: 507-518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous