Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments - PubMed (original) (raw)
Comparative Study
Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments
Daniel A Nicholson et al. J Neurosci. 2004.
Abstract
A central problem in the neurobiology of normal aging is why learning is preserved in some aged individuals yet impaired in others. To investigate this issue, we examined whether age-related deficits in spatial learning are associated with a reduction in postsynaptic density (PSD) area in hippocampal excitatory synapses (i.e., with a structural modification that is likely to have a deleterious effect on synaptic function). A hippocampus-dependent version of the Morris water maze task was used to separate Long-Evans male rats into young adult, aged learning-unimpaired, and equally aged learning-impaired groups. Axospinous synapses from the CA1 stratum radiatum were analyzed using systematic random sampling and serial section analyses. We report that aged learning-impaired rats exhibit a marked ( approximately 30%) and significant reduction in PSD area, whereas aged learning-unimpaired rats do not. The observed structural alteration involves a substantial proportion of perforated synapses but is not observed in nonperforated synapses. These findings support the notion that many hippocampal perforated synapses become less efficient in aged learning-impaired rats, which may contribute to cognitive decline during normal aging.
Figures
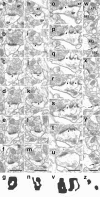
Figure 1.
Morphological subtypes of axospinous synapses from the rat CA1 stratum radiatum classified according to their PSD configuration. Electron micrographs of consecutive sections that, respectively, demonstrate a synapse with a fenestrated (_a_-f), horseshoe-shaped (_h_-m), or segmented (_o_-u) PSD, as well as two synapses (black and white arrows) with nonperforated PSDs (_w_-y). All micrographs containing PSD profiles are presented in each case. The synapses illustrated in _a_-_f, h_-m, or _o_-u belong to the perforated synaptic category because they exhibit a discontinuity(s) or perforation(s) in PSD profiles seen in consecutive sections (arrowheads). In contrast, nonperforated synapses show exclusively continuous PSD profiles in w-y. The presynaptic and postsynaptic elements of each synapse are labeled in a, h, o, and w by AT (axon terminal) and SP (spine). Two-dimensional reconstructions of PSD plates show their fenestrated (g), horseshoe-shaped (n), segmented (v), or nonperforated (z) configuration. Scale bars, 0.5 μm.
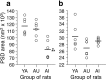
Figure 2.
The area of PSDs in axospinous synapses from the CA1 stratum radiatum of YA, AU, and AI rats. Symbols represent the values for individual rats. Horizontal lines indicate the group means. a, Perforated PSDs have a significantly smaller area in AI rats relative to either YA (*) or AU (**) rats, whereas the latter two groups of animals do not differ significantly on this measure. The means ± SD for the YA, AU, and AI groups are 117.2 ± 12.3, 112.5 ± 11.7, and 82.5 ± 13.8 nm2× 103, respectively. b, Nonperforated PSD areas are not significantly different among the three groups of rats. The means ± SD for the YA, AU, and AI groups are 30.3 ± 3.8, 26.9 ± 2.4, and 29.0 ± 1.0 nm2× 103, respectively. Note that the scale of the ordinate axis differs from that in a.
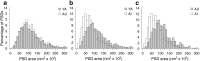
Figure 3.
Distribution of perforated PSDs with respect to their area in YA, AU, and AI rats. a, YA and AU rats have almost identical distributions. b, c, The distribution in AI rats is skewed toward smaller PSD areas compared with YA (b) and AU (c) rats.
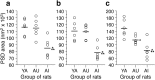
Figure 4.
The area of fenestrated, horseshoe-shaped, and segmented PSDs in the YA, AU, and AI groups. Symbols represent the values for individual rats. Horizontal lines indicate the group means. a, The fenestrated PSD area is significantly diminished in the AI group relative to the YA (*) and AU (**) groups. The means ± SD for the YA, AU, and AI groups are 116.0 ± 12.0, 114.5 ± 13.9, and 84.8 ± 14.3 nm2× 103, respectively. b, The horseshoe-shaped PSD area is significantly smaller in the AI group than in the YA (*) and AU (**) groups. The means ± SD for the YA, AU, and AI groups are 110.0 ± 14.0, 109.1 ± 10.6, and 78.2 ± 10.9 nm2× 103, respectively. c, The segmented PSD area is reduced in size in both aged groups (AU and AI) compared with the YA group (*). The AI group, however, also exhibits a significantly smaller area of segmented PSDs relative to the AU group (**). The means ± SD for the YA, AU, and AI groups are 147.9 ± 26.1, 113.8 ± 12.4, and 82.5 ± 26.8 nm2× 103, respectively.
Similar articles
- Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum.
Geinisman Y, Ganeshina O, Yoshida R, Berry RW, Disterhoft JF, Gallagher M. Geinisman Y, et al. Neurobiol Aging. 2004 Mar;25(3):407-16. doi: 10.1016/j.neurobiolaging.2003.12.001. Neurobiol Aging. 2004. PMID: 15123345 - Differential expression of PSD proteins in age-related spatial learning impairments.
Nyffeler M, Zhang WN, Feldon J, Knuesel I. Nyffeler M, et al. Neurobiol Aging. 2007 Jan;28(1):143-55. doi: 10.1016/j.neurobiolaging.2005.11.003. Epub 2005 Dec 28. Neurobiol Aging. 2007. PMID: 16386336 - Age-related synaptic changes in the CA1 stratum radiatum and spatial learning impairment in rats.
Long LH, Liu RL, Wang F, Liu J, Hu ZL, Xie N, Jin Y, Fu H, Chen JG. Long LH, et al. Clin Exp Pharmacol Physiol. 2009 Jul;36(7):675-81. doi: 10.1111/j.1440-1681.2008.05132.x. Epub 2008 Dec 11. Clin Exp Pharmacol Physiol. 2009. PMID: 19594553 - Age-dependent alterations in hippocampal synaptic plasticity: relation to memory disorders.
deToledo-Morrell L, Geinisman Y, Morrell F. deToledo-Morrell L, et al. Neurobiol Aging. 1988 Sep-Dec;9(5-6):581-90. doi: 10.1016/s0197-4580(88)80117-9. Neurobiol Aging. 1988. PMID: 3062469 Review. - Spatial Navigation (Water Maze) Tasks.
Terry AV Jr. Terry AV Jr. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 13. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 13. PMID: 21204326 Free Books & Documents. Review.
Cited by
- Altered network timing in the CA3-CA1 circuit of hippocampal slices from aged mice.
Kanak DJ, Rose GM, Zaveri HP, Patrylo PR. Kanak DJ, et al. PLoS One. 2013 Apr 8;8(4):e61364. doi: 10.1371/journal.pone.0061364. Print 2013. PLoS One. 2013. PMID: 23593474 Free PMC article. - Altered expression of ionotropic L-Glutamate receptors in aged sensory neurons of Aplysia californica.
Greer JB, Mager EM, Fieber LA. Greer JB, et al. PLoS One. 2019 May 23;14(5):e0217300. doi: 10.1371/journal.pone.0217300. eCollection 2019. PLoS One. 2019. PMID: 31120976 Free PMC article. - Intra-hippocampal D-cycloserine rescues decreased social memory, spatial learning reversal, and synaptophysin levels in aged rats.
Portero-Tresserra M, Martí-Nicolovius M, Tarrés-Gatius M, Candalija A, Guillazo-Blanch G, Vale-Martínez A. Portero-Tresserra M, et al. Psychopharmacology (Berl). 2018 May;235(5):1463-1477. doi: 10.1007/s00213-018-4858-z. Epub 2018 Feb 28. Psychopharmacology (Berl). 2018. PMID: 29492616 - Distinguishing adaptive plasticity from vulnerability in the aging hippocampus.
Gray DT, Barnes CA. Gray DT, et al. Neuroscience. 2015 Nov 19;309:17-28. doi: 10.1016/j.neuroscience.2015.08.001. Epub 2015 Aug 6. Neuroscience. 2015. PMID: 26255677 Free PMC article. Review. - Ultrastructural analyses in the hippocampus CA1 field in Shank3-deficient mice.
Uppal N, Puri R, Yuk F, Janssen WG, Bozdagi-Gunal O, Harony-Nicolas H, Dickstein DL, Buxbaum JD, Hof PR. Uppal N, et al. Mol Autism. 2015 Jun 30;6:41. doi: 10.1186/s13229-015-0036-x. eCollection 2015. Mol Autism. 2015. PMID: 26137200 Free PMC article.
References
- Amaral DG, Witter MP (1995) Hippocampal formation. In: The rat nervous system, Ed 2 (Paxinos G, ed), pp 443-493. New York: Academic.
- Baude A, Nusser Z, Molnár E, McIlhinney RA, Somogyi P (1995) High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience 69: 1031-1055. - PubMed
- Bredt DS, Nicoll RA (2003) AMPA receptor trafficking at excitatory synapses. Neuron 40: 361-379. - PubMed
- Cursio CA, Buell SJ, Coleman PD (1982) Morphology of the aging nervous system: not all downhill. In: Advances in neurogerontology, Vol III (Mortimer JA, Pirozzolo FG, Maletta GJ, eds), pp 7-35. New York: Praeger.
- Desmond NL, Weinberg RJ (1998) Enhanced expression of AMPA receptor protein at perforated axospinous synapses. NeuroReport 9: 857-860. - PubMed
Publication types
MeSH terms
Grants and funding
- P30 AG13854/AG/NIA NIH HHS/United States
- R01 AG17139/AG/NIA NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- P01 AG09973/AG/NIA NIH HHS/United States
- P01 AG009973/AG/NIA NIH HHS/United States
- R01 AG017139/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous