Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease - PubMed (original) (raw)
Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease
Josep M Canals et al. J Neurosci. 2004.
Abstract
The mechanism that controls the selective vulnerability of striatal neurons in Huntington's disease is unclear. Brain-derived neurotrophic factor (BDNF) protects striatal neurons and is regulated by Huntingtin through the interaction with the neuron-restrictive silencer factor. Here, we demonstrate that the downregulation of BDNF by mutant Huntingtin depends on the length and levels of expression of the CAG repeats in cell cultures. To analyze the functional effects of these changes in BDNF in Huntington's disease, we disrupted the expression of bdnf in a transgenic mouse model by cross-mating bdnf(+/ -) mice with R6/1 mice. Thus, we compared transgenic mice for mutant Huntingtin with different levels of BDNF. Using this double mutant mouse line, we show that the deficit of endogenous BDNF modulates the pathology of Huntington's disease. The decreased levels of this neurotrophin advance the onset of motor dysfunctions and produce more severe uncoordinated movements. This behavioral pathology correlates with the loss of striatal dopamine and cAMP-regulated phosphoprotein-32-positive projection neurons. In particular, the insufficient levels of BDNF cause specific degeneration of the enkephalinergic striatal projection neurons, which are the most affected cells in Huntington's disease. This neuronal dysfunction can specifically be restored by administration of exogenous BDNF. Therefore, the decrease in BDNF levels plays a key role in the specific pathology observed in Huntington's disease by inducing dysfunction of striatal enkephalinergic neurons that produce severe motor dysfunctions. Hence, administration of exogenous BDNF may delay or stop illness progression.
Figures
Figure 1.
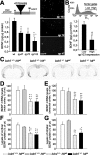
The number of CAG repeats and the levels of mutant htt expression modulate the expression of BDNF. A, ELISA for BDNF was performed on the culture media from wt M213 cells and subclones expressing exon 1 of mutant htt with 47 (qp47), 72 (qp72), and 103 CAG/CAA repeats (qp103). The expression of this neurotrophin decreases as the number of repeats is longer. The scheme at the top shows the structure of qp constructs, which expresses a fusion protein of the first 90 amino acids of the mutant htt and the EGFP. The right panels are photomicrographs of transfected cells with the qp47, 72, and 103 constructs, which show the mutant htt inclusions. CMV, Cytomegalovirus. Scale bar, 50 μm. B, Transfected cells that express different levels of qp103 were collected by cell sorting (inset shows sorting gates), and BDNF levels were assessed. The amount of BDNF in culture media is inversely proportional to the levels of expression of qp103. ***p<0.001 compared with wt cells; +p<0.05 compared with qp47 or qp72; ##p < 0.005 compared with low levels of qp103 expression. C-G, BDNF levels are not affected by the mutant exon 1 of Htt in R6/1 (bdnf+/+ httm). C-E, In situ hybridization demonstrates that mutant htt does not change the levels of bdnf expression either in the motor cortex (D) or in the sensorial cortex (E). C, A Representative coronal section (bregma, +1.1 mm) of bdnf in situ hybridization of the four genotypes analyzed. F, G, In addition, the levels of BDNF protein detected by ELISA are not modified by mutant htt in the cortex (F) or in the striatum (G). The only changes in mRNA or protein are detected in bdnf+/- mice with or without mutant htt (C-G). *p < 0.05, **p < 0.005, and ***p < 0.001 compared with wt mice (bdnf+/+ httwt); ++p < 0.005 and +++p < 0.001 compared with R6/1 mice (bdnf+/+ httm).
Figure 2.
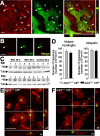
Sequestering wt Htt downregulates BDNF. A, Within nontransfected neural stem cells derived from the striatum (M213; d), wt Htt localizes in endocytic structures (open arrowheads). This localization is also detected in cells transfected with the exon 1 of htt with 72 repeats (qp72) that do not present aggregates (c) or that present small aggregates (b). However, in cells that express the qp72 construct and present large aggregates (a), wt Htt is only detected into the aggregates (filled arrowheads). We used the monoclonal antibody Mab2166 (Chemicon) to detect wt Htt (red; wt-Htt), whereas the mutant Htt was detected by the endogenous EGFP of the fusion protein (green; m-Htt). B, No signal for wt Htt was detected in the aggregates (filled arrowheads) in experiments avoiding the primary antibody. C, Western blot showing the levels of wt Htt in the cerebral cortex (Ctx) and in the striatum (Str) from R6/1 animals at 20 weeks (lanes 1-3) or at 30 weeks (lanes 4-6) or from wild-type mice at 30 weeks (lanes 7-9). Note that there are no changes in the levels of wt Htt (Htt) in R6/1. The levels of α-tubulin (Tub) are shown as a loading control. D-F, The levels of BDNF in mutant htt mice do not modify the number or morphological aspects of the intranuclear inclusions. Animals with mutant htt had intranuclear inclusions, which can be detected by mutant Htt or ubiquitin immunohistochemistry. D, The same density of aggregates was detected in both immunostaining patterns per genotype. In addition, similar results are shown in both R6/1 mice (bdnf+/+ httm) and bDM (bdnf+/ - httm), without significant differences between the two groups. E, F, Double immunohistochemistry against NeuN (red) and ubiquitin (green) demonstrated the same intranuclear location in both genotypes, containing mutant htt, with normal levels of BDNF (R6/1 mice; E) or with lower bdnf expression (bDM; F). Scale bars: A, 10 μm; B, 15 μm; (in E) E, F, 10 μm.
Figure 3.
Lower BDNF levels cause earlier onset and more severe motor dysfunctions in mutant htt mice. A-C, bDM (bdnf+/- httm) show more deficits on the rotarod than R6/1 mice (bdnf+/+ httm) tested at 16 rpm (A), 24 rpm (B), or 32 rpm (C) (p < 0.001; F-Wald test). +p < 0.05 and ++p < 0.005 compared with R6/1 mice (bdnf+/+ httm). bDM show earlier symptoms at all revolutions assayed, and the onset of motor deficits (expressed as the first significant time point with respect to control animals; A-C, arrowhead) showed advances from 6-8 weeks with respect to R6/1 mice (bdnf+/+ httm; A-C). D, In addition, bDM show more severe symptoms. The total number of accumulated falls in the period examined is significantly different between mutant htt mice with normal or low levels of BDNF. E, During early stages, the differences in the severity of motor deficits (measured as the total number of falls up to the age of 16 weeks) between R6/1 mice and bDM are very large. D, E, ***p < 0.001 compared with wt mice (bdnf+/+ httwt); +p < 0.05 and ++p < 0.005 compared with R6/1 mice (bdnf+/+ httm); ##p < 0.005 and ###p < 0.001 compared with bdnf heterozygous mice (bdnf+/ - httwt).
Figure 4.
BDNF levels control motor coordination in mutant htt mice. A-D, The decrease of bdnf in mutant htt mice produces an uncoordinated walking footprint pattern. We analyzed the following four parameters of footprints: A, the number of steps; B, the distance between two consecutive steps; C, D, the distance between right and left frontbase and hindbase path, respectively. bDM shows differences in the number and distance of steps (A, B) and in the frontbase width (C). No differences were found in the hindbase pattern (D). *p < 0.05, **p < 0.005, and ***p < 0.001 compared with wt mice (bdnf+/+ httwt); +p < 0.05 and +++p < 0.001 compared with R6/1 mice (bdnf+/+ httm); #p < 0.05, ##p < 0.005, and ###p < 0.001 compared with _bdnf_heterozygous mice (bdnf+/ - httwt). E, The levels of BDNF do not affect the mouse's ability to walk on a wire rod. Although we did not find statistical differences between groups, animals with mutant htt and with normal or lower levels of BDNF had a higher tendency to fall off the rod. The distance covered by the animals on the rod also shows the same effect, with shorter runs in those animals expressing mutant htt.
Figure 5.
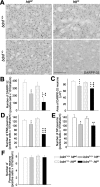
Reduction of BDNF levels in mutant htt mice produces neuronal loss in the striatum. A, Representative sections of DARPP-32 immunohistochemistry on wt mice (bdnf+/+ httwt), R6/1 mice (bdnf+/+ httm), bdnf heterozygous mice (bdnf+/ - httwt), and bDM (bdnf+/ - httm). B, C, We used DARPP-32 immunostaining to measure the volume of the cerebral cortex (B) and the striatum (C). B, The cerebral cortex shows the same atrophy in both genotypes with mutant htt. C, In contrast, the striatal volume is significantly lower in bDM with respect to R6/1 mice, indicating that the insufficient levels of this neurotrophin produce more severe striatal degeneration. D, E, At 30 weeks, bDM have striatal neuronal loss, counted by neuronal morphology in cresyl violet staining (D) or by NeuN immunohistochemistry (E). No decrease in neurons was detected in other genotypes. F, G, In n3DM (nt-3+/- httm), the effects observed result from the addition of striatal and cortical reduction observed in individual genotypes, mutant htt and nt-3+/-. H, I, No differences in cell loss were observed between _nt_-3 heterozygous with or without mutant htt. Both genotypes (nt-3+/- httwt and nt-3+/- httm) present a ∼25% reduction. *p < 0.05, **p < 0.005, and ***p < 0.001 compared with wt mice (bdnf+/+ httwt or nt-3+/+ httwt); +p < 0.05 and +++p < 0.001 compared with bdnf+/+ httm or nt-3+/+ httm mice; #p < 0.05, ##p < 0.005, and ###p < 0.001 compared with bdnf or nt-3 heterozygous mice, respectively.
Figure 6.
The striatal cells lost in bDM are enkephalin-positive neurons. A-C, At 30 weeks of age, bDM (bdnf+/- httm) had great atrophy of medium-sized striatal projection neurons, as demonstrated by DARPP-32 immunostaining. The number of DARPP-32-positive neurons decreased in all mutant htt mice (B). However, the lowest number of DARPP-32-positive neurons was detected in bDM. C, The cross-sectional area of DARPP-32 neurons decreased in all animals that express mutant htt, although the area is significantly smaller in bDM.D, E,The two neuronal subpopulations of medium-sized spiny neurons in the striatum were characterized by insitu hybridization. D, The number of enkephalin-positive neurons (ENK) was dramatically less in bDM. The number of ENK-positive neurons was also less in R6/1 mice (bdnf+/+ httm) than in wt mice but only by a small amount. E, The differences between the two mutant htt groups are statistically significant. In contrast, the decrease in substance P-positive neurons (SP) is similar in R6/1 mice (bdnf+/+ httm) and bDM (bdnf+/- httm),without significant differences. F, We also analyzed the number of striatal interneurons by parvalbumin immunohistochemistry. No differences were found between the four genotypes analyzed. *p < 0.05, **p < 0.005, and ***p < 0.001 compared with wt mice (bdnf+/+ httwt); +p<0.05 and +++p<0.001 compared with R6/1 mice (bdnf+/+ httm);#p<0.05,##p<0.005,and###p<0.001 compared with bdnf heterozygous mice (bdnf+/- httwt). Scale bar, 100 μm.
Figure 7.
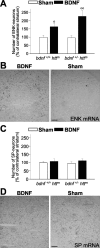
Exogenous BDNF restores the enkephalin loss in mutant htt mice. A, B, Continuous intrastriatal administration of BDNF (4.5 μg per day) during 1 week produces an increase in the number of enkephal in-positive cells (ENK). This effect may be caused by the enhancement in the enkephalin mRNA levels in both R6/1 mice (bdnf+/+ httm) and bDM (bdnf+/ - httm). C, D, However, intrastriatal administration of BDNF in the same animals does not modify the levels of substance P-positive neurons (SP). No differences were observed in PBS-infused animals (sham) with respect to untreated animals, either in enkephalin- or in substance P-positive neurons (data not shown). B, D, Representative photomicrographs of in situ hybridization for enkephalin (ENK) and substance P (SP) in bDM injected with BDNF or PBS (Sham). Note that after BDNF treatment, the enkephalin-positive neurons were detected around the injection site but not in the injection core (B). ○p < 0.05 and ○○p < 0.005 compared with sham-operated mice (Student's t test). Scale bars: B, D, 100 μm.
Similar articles
- Brain-derived neurotrophic factor modulates dopaminergic deficits in a transgenic mouse model of Huntington's disease.
Pineda JR, Canals JM, Bosch M, Adell A, Mengod G, Artigas F, Ernfors P, Alberch J. Pineda JR, et al. J Neurochem. 2005 Jun;93(5):1057-68. doi: 10.1111/j.1471-4159.2005.03047.x. J Neurochem. 2005. PMID: 15934928 - Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease.
Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, Timmusk T, Sipione S, Cattaneo E. Zuccato C, et al. Science. 2001 Jul 20;293(5529):493-8. doi: 10.1126/science.1059581. Epub 2001 Jun 14. Science. 2001. PMID: 11408619 - Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington's disease phenotypes in mice.
Gharami K, Xie Y, An JJ, Tonegawa S, Xu B. Gharami K, et al. J Neurochem. 2008 Apr;105(2):369-79. doi: 10.1111/j.1471-4159.2007.05137.x. Epub 2007 Dec 12. J Neurochem. 2008. PMID: 18086127 Free PMC article. - [Huntington's disease: intracellular signaling pathways and neuronal death].
Humbert S, Saudou F. Humbert S, et al. J Soc Biol. 2005;199(3):247-51. doi: 10.1051/jbio:2005026. J Soc Biol. 2005. PMID: 16471265 Review. French. - Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington's disease.
Alberch J, Pérez-Navarro E, Canals JM. Alberch J, et al. Brain Res Bull. 2002 Apr;57(6):817-22. doi: 10.1016/s0361-9230(01)00775-4. Brain Res Bull. 2002. PMID: 12031278 Review.
Cited by
- Human immunodeficiency virus-associated dementia: a link between accumulation of viral proteins and neuronal degeneration.
Mocchetti I, Bachis A, Esposito G, Turner SR, Taraballi F, Tasciotti E, Paige M, Avdoshina V. Mocchetti I, et al. Curr Trends Neurol. 2014;8:71-85. Curr Trends Neurol. 2014. PMID: 26069421 Free PMC article. - Rescuing the Corticostriatal Synaptic Disconnection in the R6/2 Mouse Model of Huntington's Disease: Exercise, Adenosine Receptors and Ampakines.
Cepeda C, Cummings DM, Hickey MA, Kleiman-Weiner M, Chen JY, Watson JB, Levine MS. Cepeda C, et al. PLoS Curr. 2010 Sep 20;2:RRN1182. doi: 10.1371/currents.RRN1182. PLoS Curr. 2010. PMID: 20877458 Free PMC article. - Expression profiling of Huntington's disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration.
Strand AD, Baquet ZC, Aragaki AK, Holmans P, Yang L, Cleren C, Beal MF, Jones L, Kooperberg C, Olson JM, Jones KR. Strand AD, et al. J Neurosci. 2007 Oct 24;27(43):11758-68. doi: 10.1523/JNEUROSCI.2461-07.2007. J Neurosci. 2007. PMID: 17959817 Free PMC article. - Nolz1 promotes striatal neurogenesis through the regulation of retinoic acid signaling.
Urbán N, Martín-Ibáñez R, Herranz C, Esgleas M, Crespo E, Pardo M, Crespo-Enríquez I, Méndez-Gómez HR, Waclaw R, Chatzi C, Alvarez S, Alvarez R, Duester G, Campbell K, de Lera AR, Vicario-Abejón C, Martinez S, Alberch J, Canals JM. Urbán N, et al. Neural Dev. 2010 Aug 24;5:21. doi: 10.1186/1749-8104-5-21. Neural Dev. 2010. PMID: 20735826 Free PMC article. - The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington's disease mouse model.
Peng Q, Masuda N, Jiang M, Li Q, Zhao M, Ross CA, Duan W. Peng Q, et al. Exp Neurol. 2008 Mar;210(1):154-63. doi: 10.1016/j.expneurol.2007.10.015. Epub 2007 Nov 9. Exp Neurol. 2008. PMID: 18096160 Free PMC article.
References
- Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P (2003) BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development 130: 1479-1491. - PubMed
- Alberch J, Perez-Navarro E, Canals JM (2002) Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington's disease. Brain Res Bull 57: 817-822. - PubMed
- Alberch J, Perez-Navarro E, Canals JM (2004) Neurotrophic factors in Huntington's disease. Prog Brain Res 146: 195-229. - PubMed
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ (1997) Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389: 856-860. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases