Proapoptotic N-truncated BCL-xL protein activates endogenous mitochondrial channels in living synaptic terminals - PubMed (original) (raw)
. 2004 Sep 14;101(37):13590-5.
doi: 10.1073/pnas.0401372101. Epub 2004 Sep 1.
John A Hickman, Mushtaque Chachar, Brian M Polster, Teresa A Brandt, Yihru Fannjiang, Iva Ivanovska, Gorka Basañez, Kathleen W Kinnally, Joshua Zimmerberg, J Marie Hardwick, Leonard K Kaczmarek
Affiliations
- PMID: 15342906
- PMCID: PMC518799
- DOI: 10.1073/pnas.0401372101
Proapoptotic N-truncated BCL-xL protein activates endogenous mitochondrial channels in living synaptic terminals
Elizabeth A Jonas et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Neuronal death is often preceded by functional alterations at nerve terminals. Anti- and proapoptotic BCL-2 family proteins not only regulate the neuronal death pathway but also affect excitability of healthy neurons. We found that exposure of squid stellate ganglia to hypoxia, a death stimulus for neurons, causes a cysteine protease-dependent loss of full-length antiapoptotic BCL-xL, similar to previous findings in mammalian cells. Therefore, to determine the direct effect of the naturally occurring proapoptotic cleavage product of BCL-xL on mitochondria, recombinant N-truncated BCL-xL was applied to mitochondria inside the squid presynaptic terminal and to purified mitochondria isolated from yeast. N-truncated BCL-xL rapidly induced large multi-conductance channels with a maximal conductance significantly larger than those produced by full-length BCL-xL. This activity required the hydrophobic C terminus and the BH3 domain of BCL-xL. Moreover, N-truncated BCL-xL failed to produce any channel activity when applied to plasma membranes, suggesting that a component of the mitochondrial membrane is necessary for its actions. Consistent with this idea, the large channels induced by N-truncated BCL-xL are inhibited by NADH and require the presence of VDAC, a voltage-dependent anion channel present in the outer mitochondrial membrane. These observations suggest that the mitochondrial channels specific to full-length and N-truncated BCL-xL contribute to their opposite effects on synaptic transmission, and are consistent with their opposite effects on the cell death pathway.
Figures
Fig. 1.
Mitochondrial channel activity produced by ΔN BCL-xL. (a) Channel activity recorded at –100 mV using pipettes containing 8.0 μg/ml ΔN BCL-xL. (b) I-V relations for ΔN BCL-xL-induced activity, showing examples of intermediate and large conductances. (c) Cumulative distribution of probability of channel activity of different conductances in experiments with control intracellular solution, ΔN BCL-xL, or full-length BCL-xL. Histograms combining all experiments show the probability of closed channels, activity <180 pS (Small), activity between 180 and 760 pS (Intermediate), and activity >760 pS (Large). For controls, n = 16; for ΔN BCL-xL, n = 6; for FL BCL-xL, n = 4 (probabilities relative to control *, P < 0.025; **, P < 0.005). (d) Time course of transitions of channel opening to different conductances. Shown are transitions in recordings with 8.0 μg/ml ΔN BCL-xL (Right) or full-length BCL-xL (Left) in the patch pipette solution. Openings were divided into the three groups as above. Probability of occurrence of openings in each group within successive 10-s recording periods is plotted as a function of time.
Fig. 2.
Specificity of channel formation by ΔN BCL-xL. (a) Activity on a mitochondrial membrane recorded at –100 mV using a pipette containing 8.0 μg/ml ΔN BCL-xL. C marks the closed state. (b) Lack of activity in the presence of the BH3 domain mutant ΔN Δ90-92 BCL-xL. Patch potential was –100 mV. (c) Lack of effect of ΔN BCL-xL in cell-attached plasma membrane recordings of molluscan neurons. Endogenous small-conductance plasma membrane channel activity (42) can be detected. Patch potential was –160 mV.
Fig. 3.
Effects of NADH on multiconductance channel activity produced by ΔN BCL-xL. (a) Bar graphs show distribution of openings for patches exposed to 8.0 μg/ml ΔN BCL-xL with 2 mM NADH (n = 10). (b) Lack of effect of NADH on the release of fluorescent indicator (ANTS) from artificial lipid vesicles in the presence of ΔN BCL-xL. Lipid and protein concentrations were 50 μM and 50 nM, respectively.
Fig. 4.
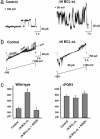
Actions of ΔN BCL-xL on channel activity in isolated mitochondria from wild-type yeast and those lacking the POR1 gene (ΔPOR1). (a) Typical channel activity in wild-type yeast mitochondria in the absence (Left) or presence (Right) of 8.0 μg/ml ΔN BCL-xL. (b) Channel activity during a 10-s ramp from –100 to +100 mV. Holding potential before and after the ramp was 0 mV. (Left) Response in the absence of ΔN BCL-xL. As expected for the VDAC, increased channel openings occur near the center of the ramp. The slope of the closed state is indicated, as is the slope of the subconductance state (sub) that persists at positive and negative potentials. (Right) Response with 8.0 μg/ml ΔN BCL-xL. (c) Bar graphs show the maximal channel conductance recorded in the absence or presence of 8.0 μg/ml ΔN BCL-xL, or of ΔN BCL-xL with 2 mM NADH. Shown are data for wild-type mitochondria (Left) and ΔPOR1 mutant mitochondria (Right).
Fig. 5.
zVAD prevents loss of full-length BCL-xL protein during hypoxia. (a) Immunoblot of untreated or hypoxia-treated (60 min) squid stellate ganglia incubated in the absence or presence of zVAD (100 μM) by using anti-chicken BCL-x antibody. Equal loading was verified by comparing a crossreacting band near the top of the gel. (b) Immunoblot of recombinant human BCL-xL protein and of mitochondria (mito) purified from hypoxic (20 min) squid stellate ganglia (± zVAD) by using anti-Bcl-x antibody (Top). (Middle) Immunoblots using the same antibody preadsorbed with recombinant human BCL-xL protein. (Bottom) Immunoblot analysis using an anti-VDAC antibody.
Similar articles
- Regulation of synaptic transmission by mitochondrial ion channels.
Jonas E. Jonas E. J Bioenerg Biomembr. 2004 Aug;36(4):357-61. doi: 10.1023/B:JOBB.0000041768.11006.90. J Bioenerg Biomembr. 2004. PMID: 15377872 Review. - Actions of BAX on mitochondrial channel activity and on synaptic transmission.
Jonas EA, Hardwick JM, Kaczmarek LK. Jonas EA, et al. Antioxid Redox Signal. 2005 Sep-Oct;7(9-10):1092-100. doi: 10.1089/ars.2005.7.1092. Antioxid Redox Signal. 2005. PMID: 16115013 - Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC.
Shimizu S, Narita M, Tsujimoto Y. Shimizu S, et al. Nature. 1999 Jun 3;399(6735):483-7. doi: 10.1038/20959. Nature. 1999. PMID: 10365962 - Bax and Bcl-xL independently regulate apoptotic changes of yeast mitochondria that require VDAC but not adenine nucleotide translocator.
Shimizu S, Shinohara Y, Tsujimoto Y. Shimizu S, et al. Oncogene. 2000 Sep 7;19(38):4309-18. doi: 10.1038/sj.onc.1203788. Oncogene. 2000. PMID: 10980606 - Control of mitochondrial permeability by Bcl-2 family members.
Sharpe JC, Arnoult D, Youle RJ. Sharpe JC, et al. Biochim Biophys Acta. 2004 Mar 1;1644(2-3):107-13. doi: 10.1016/j.bbamcr.2003.10.016. Biochim Biophys Acta. 2004. PMID: 14996495 Review.
Cited by
- MAC and Bcl-2 family proteins conspire in a deadly plot.
Dejean LM, Ryu SY, Martinez-Caballero S, Teijido O, Peixoto PM, Kinnally KW. Dejean LM, et al. Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):1231-8. doi: 10.1016/j.bbabio.2010.01.007. Epub 2010 Jan 18. Biochim Biophys Acta. 2010. PMID: 20083086 Free PMC article. Review. - Bcl-xL inhibitor ABT-737 reveals a dual role for Bcl-xL in synaptic transmission.
Hickman JA, Hardwick JM, Kaczmarek LK, Jonas EA. Hickman JA, et al. J Neurophysiol. 2008 Mar;99(3):1515-22. doi: 10.1152/jn.00598.2007. Epub 2007 Dec 26. J Neurophysiol. 2008. PMID: 18160428 Free PMC article. - Reflections on VDAC as a voltage-gated channel and a mitochondrial regulator.
Mannella CA, Kinnally KW. Mannella CA, et al. J Bioenerg Biomembr. 2008 Jun;40(3):149-55. doi: 10.1007/s10863-008-9143-0. J Bioenerg Biomembr. 2008. PMID: 18648913 Review. - BDNF signaling: Harnessing stress to battle mood disorder.
Licznerski P, Jonas EA. Licznerski P, et al. Proc Natl Acad Sci U S A. 2018 Apr 10;115(15):3742-3744. doi: 10.1073/pnas.1803645115. Epub 2018 Mar 28. Proc Natl Acad Sci U S A. 2018. PMID: 29592951 Free PMC article. No abstract available. - High-resolution analysis of the conformational transition of pro-apoptotic Bak at the lipid membrane.
Sperl LE, Rührnößl F, Schiller A, Haslbeck M, Hagn F. Sperl LE, et al. EMBO J. 2021 Oct 18;40(20):e107159. doi: 10.15252/embj.2020107159. Epub 2021 Sep 15. EMBO J. 2021. PMID: 34523144 Free PMC article.
References
- Boise, L. H., González-Garcia, M., Postema, C. E., Ding, L., Lindstein, T., Turka, L. A., Mao, X., Nûñez, G. & Thompson, C. B. (1993) Cell 74, 597–608. - PubMed
- Krajewski, S., Krajewska, M., Shabaik, A., Wang, H. G., Irie, S., Fong, L. & Reed, J. C. (1994) Cancer Res. 54, 5501–5507. - PubMed
- Frankowski, H., Missotten, M., Fernandez, P. A., Martinou, I., Michel, P., Sadoul, R. & Martinou, J. C. (1995) NeuroReport 6, 1917–1921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS 34175/NS/NINDS NIH HHS/United States
- R01 NS034175/NS/NINDS NIH HHS/United States
- GM 57249/GM/NIGMS NIH HHS/United States
- R01 GM057249/GM/NIGMS NIH HHS/United States
- R01 NS037402/NS/NINDS NIH HHS/United States
- ND 45876/ND/ONDIEH CDC HHS/United States
- R01 NS045876/NS/NINDS NIH HHS/United States
- NS 37402/NS/NINDS NIH HHS/United States
- NS 18496/NS/NINDS NIH HHS/United States
- R37 NS045876/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials