The septin Sept5/CDCrel-1 competes with alpha-SNAP for binding to the SNARE complex - PubMed (original) (raw)
The septin Sept5/CDCrel-1 competes with alpha-SNAP for binding to the SNARE complex
Crestina L Beites et al. Biochem J. 2005.
Abstract
SNARE (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor) proteins are supposed to mediate the docking and/or fusion of the vesicle with the plasma membrane. However, it is not clearly understood how this process is regulated. In a search for potential SNARE regulators, we recently identified septin 5 (Sept5) as a novel SNARE interacting protein. Septins were first identified as filamentous proteins required for cytokinesis in yeast. Several septins have now been identified in mammals but little is known about their functions. We have previously shown that Sept5 is predominantly expressed in the brain, where it associates with vesicles and membranes through its interaction with the SNARE domain of syntaxin 1A. Furthermore, Sept5 appears to inhibit exocytosis, possibly by regulating vesicle targeting and/or fusion events. To gain insight into the role of Sept5, we have mapped the Sept5 domains important for syntaxin binding. We also investigated the ability of Sept5 to bind to syntaxin when in various protein complexes. Although Sept5 cannot bind an nSec1-syntaxin complex, it can bind syntaxin in a SNARE complex. This interaction is occluded by the binding of alpha-SNAP, suggesting that Sept5 may regulate the availability of SNARE proteins through its interaction with syntaxin and the 7 S complex.
Figures
Figure 1. Specific interaction of truncation mutants of Sept5 with syntaxin in vitro
GST alone or truncation mutants of Sept5 (0.1 nmol) were bound to glutathione–agarose and incubated with soluble recombinant syntaxin 1A (0.2 nmol). After washing, syntaxin bound to the various constructs was analysed by SDS/PAGE and immunoblotting with anti-syntaxin antibodies. Whereas GST alone or GST–Sept5 truncations of residues 1–60 and 110–213 did not appreciably bind syntaxin, the binding of the N-terminal region of Sept5 to syntaxin is stronger than that of full-length or C-terminal region of Sept5.
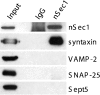
Figure 2. Sept5 does not associate with the nSec1–syntaxin complex
Detergent extract of rat brain was incubated with anti-nSec1 antibodies (lane 3) or control IgG (lane 2) as described in the Experimental section. The immunoprecipitated samples as well as 20 μg of the starting material were resolved by SDS/PAGE and immunoblotted with various antibodies as indicated.
Figure 3. Sedimentation of SNARE proteins
Rat brain P2 fractions were sedimented on a glycerol gradient (A) and then immunoprecipitated (B) with anti-syntaxin antibodies or control IgG. Although Sept5 fractionated within the 7 S region of the gradient-like syntaxin, SNAP-25 and VAMP-2, no Sept5, α-SNAP or nSec1 was immunoprecipitated with the 7 S complex. The protein markers used were BSA (4.6 S) and catalase (11.3 S).
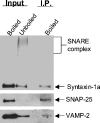
Figure 4. Core SNARE complexes containing SNAP-25 and syntaxin 1A co-immunoprecipitate with Sept5
Trition X-100 extracts of rat brain were incubated with anti-Sept5 antibodies bound to Protein A beads. Immunoprecipitated material was suspended in Laemmli sample buffer and either loaded directly or boiled (as indicated above each lane). The first two lanes of each blot represent 2% of the initial material (input) used for immunoprecipitation. The probing antibody is indicated to the right of each blot and the bands detected by these antibodies are indicated with arrows.
Figure 5. SNAREs but not SNAP–SNAREs associate with Sept5
GST-α-SNAP immobilized on glutathione–agarose beads were incubated with salt-washed detergent extracts of rat brain and washed to remove unbound material. Precipitated complexes still bound to beads were then either released by incubating with high salt and detergent (A) to yield pure SNARE complexes or thrombin cleaved to yield SNARE complexes bound to α-SNAP (B). These complexes were then sedimented on a glycerol gradient and analysed by SDS/PAGE and immunoblotting with the indicated antibodies. To determine whether Sept5 can bind to 7 S complexes in the absence of α-SNAP, the 7 S region of the gradients containing either pure SNAREs (C) or SNAP–SNAREs (D) were incubated with GST–Sept5 as described in the Experimental section. Whereas SNAREs bound to α-SNAP could not bind GST–Sept5 (D), the absence of α-SNAP allows SNAREs to bind GST–Sept5 (C). The last panel in blot (C) was overexposed to show that no α-SNAP was present. Neither complexes bound GST alone. Results in each panel are representative of three independent experiments.
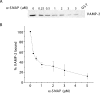
Figure 6. SNARE complexes are released from Sept5 in the presence of α-SNAP
SNARE complexes immobilized on GST–Sept5 glutathione–agarose beads were incubated with increasing concentrations of recombinant α-SNAP. SNARE complexes remaining bound to GST–Sept5 were then detected by resolving the material on SDS/PAGE followed by immunoblotting with anti-VAMP-2 antibodies to detect the presence of the SNARE complex (A). The values expressed as percentages in (B) are quantifications of the blots from three independent experiments, where 100% VAMP-2 bound represents the amount bound in the absence of α-SNAP.
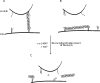
Figure 7. Models for Sept5 organization and role in secretion
(A) Septins may act as molecular tethers. By binding to 7 S complexes both on the vesicle and the plasma membrane, Sept5 and associated septins (○○) can physically restrict the movement of vesicles towards the membrane. (B) Septins may create a physical barrier to the membrane. Alternatively, septins may form networks parallel to the membrane demarcating inactive zones where SNAREs are incompetent for fusion. (C) α-SNAP displaces the septins. In either scenario, α-SNAP can then displace Sept5 from the 7 S complex, priming the SNAREs for fusion. The formation of trans complexes would then lead to exocytosis.
Similar articles
- Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis.
Amin ND, Zheng YL, Kesavapany S, Kanungo J, Guszczynski T, Sihag RK, Rudrabhatla P, Albers W, Grant P, Pant HC. Amin ND, et al. J Neurosci. 2008 Apr 2;28(14):3631-43. doi: 10.1523/JNEUROSCI.0453-08.2008. J Neurosci. 2008. PMID: 18385322 Free PMC article. - Evidence for SNARE zippering during Ca2+-triggered exocytosis in PC12 cells.
Matos MF, Mukherjee K, Chen X, Rizo J, Südhof TC. Matos MF, et al. Neuropharmacology. 2003 Nov;45(6):777-86. doi: 10.1016/s0028-3908(03)00318-6. Neuropharmacology. 2003. PMID: 14529716 - Syntaxin/Munc18 interactions in the late events during vesicle fusion and release in exocytosis.
Graham ME, Barclay JW, Burgoyne RD. Graham ME, et al. J Biol Chem. 2004 Jul 30;279(31):32751-60. doi: 10.1074/jbc.M400827200. Epub 2004 Jun 1. J Biol Chem. 2004. PMID: 15175344 - Plasma membrane targeting of exocytic SNARE proteins.
Salaün C, James DJ, Greaves J, Chamberlain LH. Salaün C, et al. Biochim Biophys Acta. 2004 Aug 23;1693(2):81-9. doi: 10.1016/j.bbamcr.2004.05.008. Biochim Biophys Acta. 2004. PMID: 15313010 Review. - SNAREs and membrane fusion in the Golgi apparatus.
Nichols BJ, Pelham HR. Nichols BJ, et al. Biochim Biophys Acta. 1998 Aug 14;1404(1-2):9-31. doi: 10.1016/s0167-4889(98)00044-5. Biochim Biophys Acta. 1998. PMID: 9714710 Review.
Cited by
- Septin-5 and -7-IgGs: Neurologic, Serologic, and Pathophysiologic Characteristics.
Hinson SR, Honorat JA, Grund EM, Clarkson BD, Miske R, Scharf M, Zivelonghi C, Al-Lozi MT, Bucelli RC, Budhram A, Cho T, Choi E, Grell J, Lopez-Chiriboga AS, Levin M, Merati M, Montalvo M, Pittock SJ, Wilson MR, Howe CL, McKeon A. Hinson SR, et al. Ann Neurol. 2022 Dec;92(6):1090-1101. doi: 10.1002/ana.26482. Epub 2022 Aug 27. Ann Neurol. 2022. PMID: 36053822 Free PMC article. - Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis.
Amin ND, Zheng YL, Kesavapany S, Kanungo J, Guszczynski T, Sihag RK, Rudrabhatla P, Albers W, Grant P, Pant HC. Amin ND, et al. J Neurosci. 2008 Apr 2;28(14):3631-43. doi: 10.1523/JNEUROSCI.0453-08.2008. J Neurosci. 2008. PMID: 18385322 Free PMC article. - The emerging functions of septins in metazoans.
Saarikangas J, Barral Y. Saarikangas J, et al. EMBO Rep. 2011 Oct 28;12(11):1118-26. doi: 10.1038/embor.2011.193. EMBO Rep. 2011. PMID: 21997296 Free PMC article. Review. - Animal models of gene-environment interaction in schizophrenia: A dimensional perspective.
Ayhan Y, McFarland R, Pletnikov MV. Ayhan Y, et al. Prog Neurobiol. 2016 Jan;136:1-27. doi: 10.1016/j.pneurobio.2015.10.002. Epub 2015 Oct 25. Prog Neurobiol. 2016. PMID: 26510407 Free PMC article. Review. - Synaptic and Gene Regulatory Mechanisms in Schizophrenia, Autism, and 22q11.2 Copy Number Variant-Mediated Risk for Neuropsychiatric Disorders.
Forsyth JK, Nachun D, Gandal MJ, Geschwind DH, Anderson AE, Coppola G, Bearden CE. Forsyth JK, et al. Biol Psychiatry. 2020 Jan 15;87(2):150-163. doi: 10.1016/j.biopsych.2019.06.029. Epub 2019 Jul 11. Biol Psychiatry. 2020. PMID: 31500805 Free PMC article.
References
- Jahn R., Lang T., Südhof T. C. Membrane fusion. Cell (Cambridge, Mass.) 2003;112:519–533. - PubMed
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature (London) 1993;362:318–324. - PubMed
- Haas A. NSF–fusion and beyond. Trends Cell Biol. 1998;8:471–473. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases