Loss of flight and associated neuronal rhythmicity in inositol 1,4,5-trisphosphate receptor mutants of Drosophila - PubMed (original) (raw)
Loss of flight and associated neuronal rhythmicity in inositol 1,4,5-trisphosphate receptor mutants of Drosophila
Santanu Banerjee et al. J Neurosci. 2004.
Abstract
Coordinated flight in winged insects requires rhythmic activity of the underlying neural circuit. Here, we show that Drosophila mutants for the inositol 1,4,5-trisphosphate (InsP(3)) receptor gene (itpr) are flightless. Electrophysiological recordings from thoracic indirect flight muscles show increased spontaneous firing accompanied by a loss of rhythmic flight activity patterns normally generated in response to a gentle puff of air. In contrast, climbing speed, the jump response, and electrical properties of the giant fiber pathway are normal, indicating that general motor coordination and neuronal excitability are much less sensitive to itpr mutations. All mutant phenotypes are rescued by expression of an itpr(+) transgene in serotonin and dopamine neurons. Pharmacological and immunohistochemical experiments support the idea that the InsP(3) receptor functions to modulate flight specifically through serotonergic interneurons. InsP(3) receptor action appears to be important for normal development of the flight circuit and its central pattern generator.
Figures
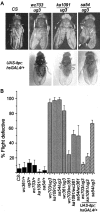
Figure 1.
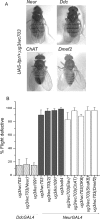
Altered wing posture and flight in viable itpr heteroallelic combinations and their rescue by a UAS-itpr+ transgene. A, Heteroallelic genotypes (itprEMS1/itprEMS2) have been designated by names of the respective alleles (ka1091, wc703, ug3, and sa54). Wild-type flies (CS) exhibit normal wing posture. In wc703/ug3 and ka1091/ug3 organisms, the wing posture is altered and is similar to down-turned wings described previously (Huang and Stern, 2002). Wing posture phenotype is 100% penetrant for the heteroallelic combinations shown. sa54/ug3 organisms have an altered wing posture and crumpled wing margins (arrowhead). hsGAL4 (single copy on second chromosome) driven basal expression of an UAS-itpr+ transgene (single copy on X chromosome/) (Venkatesh et al., 2001) at 25°C rescues the wing posture in all mutant genotypes tested. A strict quantification of the wing phenotype was not possible because it cannot be distinguished reliably in anesthetized flies. B, Genotypes tested for flight are indicated under each bar in the graph. Flight behavior of itprEMS/+ flies is not significantly different from that of wild-type flies (CS; black bars). All wc361/itprEMS combinations have flight defects ranging from 30 to 65% (gray bars). These combinations did not show any wing posture defects (see Results). Combinations of ug3/itprEMS have severe flight defects ranging from 80 to 98% (gray bars) and defects in wing posture(A). These flight defects can be rescued by ubiquitous expression of a UAS-itpr+ transgene (hatched bars). An hsGAL4 promoter construct on the second chromosome was used to drive expression of one copy of UAS-itpr+ on the X chromosome in otherwise mutant backgrounds. In wc703/ug3 and ka1091/ug3 organisms, flight defects were rescued completely, whereas in sa54/ug3 flies, a limited rescue of flight defects was seen (see Results for details).
Figure 2.
DLMs of itpr mutants exhibit increased levels of spontaneous firing. A, DLMs of wild-type flies (CS) can express short bursts of electrical activity in the absence of either mechanical or electrical stimulation. These spontaneous bursts of action potentials are unrelated to flight and are usually associated with cleaning behavior in wild-type flies (Lee and Wu, 2002). The DLMs of itpr heteroalleles, wc703/ug3 and ka1091/ug3, exhibit an increased frequency of spontaneous firing, which is unrelated to cleaning behavior. This increased frequency was suppressed by expression of UAS-itpr+ with hsGAL4 (UAS-itpr+; hsGal4; wc703/ug3 in 5 of 5 animals and UAS-itpr+; hsGal4; ka1091/ug3 in 6 of 6 animals). DLM activity was recorded in tethered flies that were placed in a dark chamber and kept undisturbed during recording. B, Average number of spontaneous spikes recorded from flies of the indicated genotypes. Spontaneous activity was determined by counting the number of spikes that occurred over 2 min. Results from each fly are indicated by open circles. The filled bars indicate the average for all flies of the specific genotype, and error bars indicate SD (*p < 0.05; **p < 0.01).
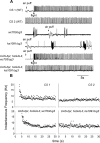
Figure 3.
Air puff-induced flight activity is absent in itpr heteroalleles. A, Flight induction by an air puff in wild-type (CS) flies as a consequence of which the DLMs exhibit a tonic firing pattern (CS1), which is maintained until cessation of flight (CS2). This flight pattern is distinct from spontaneous spiking activities (Fig. 2), and the two tend to be nonoverlapping. Air puff stimulation failed to induce a flight activity pattern in the DLMs of_itpr_ heteroalleles (wc703/ug3 and ka1091/ug3; 4 of 4 animals that were tested for each genotype) and did not interrupt the aberrant background spontaneous activities. Flight defects in InsP3R mutants were restored by the expression of the itpr+ cDNA with the hsGAL4 driver (UAS-itpr+; hsGal4; wc703/ug3;4 of 5 animals and UAS-itpr+; hsGal4; ka1091/ug3, 4 of 6 animals). B, Expression of the itpr+ cDNA restores normal frequency of tonic firing in itpr mutants. Examples of flight pattern shown in A have been plotted to show an instantaneous frequency (inversion of interspike interval) pattern. Note that the instantaneous spike frequency is high at the onset of flight and then maintained between 5 and 10 Hz.
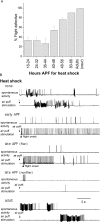
Figure 7.
Expression of the itpr gene during early pupal development is sufficient to rescue flight behavior and physiology. A, Transgenic itpr+ induction by a 1 hr heat shock at 37°C during pupal development (at the indicated hours after puparium formation) could significantly rescue flight defects in animals of UAS-itpr+; hsGAL4 (t)/+; wc703/ug3 genotype. Flies were tested in batches of 10 for each time point. n ≥ 60 for all trials. B, Normal spontaneous firing rates and flight activity patterns were restored in UAS-itpr+; hsGAL4 (t)/+ wc703/ug3 flies after a 1 hr heat shock of 37°C delivered during early pupal development (but not during adulthood). Flies obtained from pupae given a heat shock during first 2 d of pupal development at 25°C (5 of 5) displayed normal levels of spontaneous DLM spike activities and the typical flight spike pattern initiated by air puffs (early APF). In contrast, only two of four flies from pupae receiving a heat shock in the last 2 d of pupal development displayed normal levels of spontaneous DLM spike activities and the typical flight spike pattern initiated by air puffs (late APF-flier). Late-APF nonfliers did not initiate flight activity, although the abnormal spontaneous bursting was reduced in DLMs. Adult heat shock was ineffective and still showed abnormally high spontaneous bursting activity indistinguishable from that observed in control flies without heat shock. Flies were raised at 25°C.
Figure 6.
Abnormal flight behavior and physiology are observed after blocking synaptic transmission in DdcGAL4 neurons. A, Blocking synaptic transmission in DdcGAL4 (serotonin and dopamine)-positive neurons, by expression of tetanus toxin (UAS-TNT), could partially phenocopy the flight behavior defects seen in itpr mutants. Inhibition of serotonin synthesis by acute PCPA feeding of adult flies for 5 d also phenocopies flight defects seen in itpr mutants (see Materials and Methods and Results for details). n ≥ 60 for all trials. B, Independent samples of fliers (n = 54) and nonfliers (n = 160) were obtained from flies expressing UAS-TNT in the DdcGAL4 domain and examined for flight physiology. Flies in which an air puff failed to initiate flight activity (3 of 3) showed abnormally high spontaneous bursting activities in the DLMs, similar to that observed in itpr mutants. Flies that initiated flight activity on air puff stimulation (3 of 3) showed normal levels of spontaneous spiking activity. Presumably in these flies, the TNT transgene was not expressed at levels sufficient for loss of synaptic function. Normal flight activity and spontaneous firing rates were observed in control flies generated by mating flies from the DdcGal4 strain with flies carrying an inactive form of tetanus toxin, referred to as UAS-TNT(in).
Figure 8.
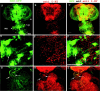
Visualization of 5-HT immunoreactivity and flight motor neurons in the adult CNS. A, D, Flight motor neurons were visualized by expressing a UAS-GFP transgene with OK6GAL4. e, Eye; tg, thoracic ganglion; ag, abdominal ganglion; mn, motoneurons. 5-HT immunoreactivity in the corresponding regions is shown in B and E. Merged images of GFP-positive motor neurons and 5-HT immunoreactivity are shown in C and _F. A_-C were visualized with a 10× objective on the MRC 1024 Bio-Rad confocal microscope. _D_-F are confocal images taken with a 60× objective of the region marked with an asterisk in A. They show flight motor neuron cell bodies (D, F, arrows) and 5-HT immunoreactive varicosities adjacent to these cell bodies (F, arrows). Colocalization of 5-HT-positive varicosities on motor neurons could be observed in individual 0.3 μm confocal sections (data not shown). _G_-I are confocal images of an adult brain-thoracic ganglion preparation expressing GFP in the DdcGAL4 domain. They were taken using a 20× objective. Arrows in G and H show DdcGAL4 and 5-HT-positive axon bundles, respectively, descending from the brain into the thoracic ganglion. Their colocalization is shown in I. Scale bars: _A_-C, 100 μm; _D_-I, 50 μm.
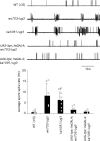
Figure 4.
Tissue and cell type-specific rescue of wing posture and flight defects in itpr heteroallelic combinations. A, Rescue of wing posture. Expression of UAS-itpr with neurGAL4 and DdcGAL4 rescues wing posture in wc703/ug3, ka1091/ug3 organisms. No rescue was observed when UAS-itpr+ expression was driven by GAL4 strains that express in all cholinergic neurons (ChATGAL4) or in all developing muscles (Dmef2GAL4) in wc703/ug3 mutant organisms. B, Rescue of flight behavior. _DdcGAL4_-mediated expression of UAS-itpr+ rescues the flight defect close to wild-type levels in itpr mutants (hatched columns). _NeurGAL4_-mediated expression of one copy of UAS-itpr+ failed to rescue flight in any combination tested (gray bars). Two copies of UAS-itpr+ in ug3/wc703(2) organisms still had no effect. Expression of UAS-itpr+ with elavGAL4 (pan-neural; has uneven levels of expression in adult neurons), ChATGAL4 (cholinergic neurons), Ok6GAL4 (motor neurons), and ShakBGAL4 (giant fiber pathway neurons), in combination with neurGAL4, failed to rescue flight to any significant level (white columns). NeurGAL4 was included for rescue of wing posture.
Figure 5.
Cell type-specific rescue of increased spontaneous firing and air puff responses. A, Expression of UAS-itpr+ in the domain of the neurGal4 driver (UAS-itpr+/+;+/+; neurGal4, ug3/wc703; 4 of 4 animals) failed to rescue either increased levels of spontaneous firing or the absence of air puff-evoked firing in the DLMs. However, DdcGAL4 driven expression of UAS-itpr+ in an itpr mutant combination (UAS-itpr+/+; DdcGal4/+; wc703/ug3) suppressed spontaneous firing (5 of 7 animals; compare with WT trace in Fig. 2) and restored normal flight activity patterns (CS1 and CS2 traces in Fig. 3) in response to air puff stimulation. B, Frequency of the firing pattern recorded from DLMs of UAS-itpr+/+; DdcGAL4 rescued itpr mutant flies is similar to that of wild-type flies (5-10 Hz) (Fig. 3). Firing frequency during spontaneous flight, in the rescued strain, is also similar to wild-type levels.
Similar articles
- Ectopic expression of a Drosophila InsP(3)R channel mutant has dominant-negative effects in vivo.
Srikanth S, Banerjee S, Hasan G. Srikanth S, et al. Cell Calcium. 2006 Feb;39(2):187-96. doi: 10.1016/j.ceca.2005.10.013. Epub 2005 Dec 1. Cell Calcium. 2006. PMID: 16325255 - Functional properties of the Drosophila melanogaster inositol 1,4,5-trisphosphate receptor mutants.
Srikanth S, Wang Z, Tu H, Nair S, Mathew MK, Hasan G, Bezprozvanny I. Srikanth S, et al. Biophys J. 2004 Jun;86(6):3634-46. doi: 10.1529/biophysj.104.040121. Biophys J. 2004. PMID: 15189860 Free PMC article. - NorpA and itpr mutants reveal roles for phospholipase C and inositol (1,4,5)- trisphosphate receptor in Drosophila melanogaster renal function.
Pollock VP, Radford JC, Pyne S, Hasan G, Dow JA, Davies SA. Pollock VP, et al. J Exp Biol. 2003 Mar;206(Pt 5):901-11. doi: 10.1242/jeb.00189. J Exp Biol. 2003. PMID: 12547945 - [Inositol 1,4,5-trisphosphate receptor (IP3 receptor)].
Mikoshiba K. Mikoshiba K. Tanpakushitsu Kakusan Koso. 1997 Feb;42(3 Suppl):224-9. Tanpakushitsu Kakusan Koso. 1997. PMID: 9162955 Review. Japanese. No abstract available. - [IP3 receptor and calcium signaling].
Yamazaki H, Michikawa T, Mikoshiba K. Yamazaki H, et al. Tanpakushitsu Kakusan Koso. 2005 Aug;50(10 Suppl):1212-9. Tanpakushitsu Kakusan Koso. 2005. PMID: 16104587 Review. Japanese. No abstract available.
Cited by
- Mutant IP3 receptors attenuate store-operated Ca2+ entry by destabilizing STIM-Orai interactions in Drosophila neurons.
Chakraborty S, Deb BK, Chorna T, Konieczny V, Taylor CW, Hasan G. Chakraborty S, et al. J Cell Sci. 2016 Oct 15;129(20):3903-3910. doi: 10.1242/jcs.191585. Epub 2016 Sep 2. J Cell Sci. 2016. PMID: 27591258 Free PMC article. - Synaptic activity in serotonergic neurons is required for air-puff stimulated flight in Drosophila melanogaster.
Sadaf S, Birman S, Hasan G. Sadaf S, et al. PLoS One. 2012;7(9):e46405. doi: 10.1371/journal.pone.0046405. Epub 2012 Sep 27. PLoS One. 2012. PMID: 23029511 Free PMC article. - The enigma of store-operated ca-entry in neurons: answers from the Drosophila flight circuit.
Hasan G, Venkiteswaran G. Hasan G, et al. Front Neural Circuits. 2010 Mar 30;4:10. doi: 10.3389/fncir.2010.00010. eCollection 2010. Front Neural Circuits. 2010. PMID: 20407638 Free PMC article. - A pupal transcriptomic screen identifies Ral as a target of store-operated calcium entry in Drosophila neurons.
Richhariya S, Jayakumar S, Abruzzi K, Rosbash M, Hasan G. Richhariya S, et al. Sci Rep. 2017 Feb 14;7:42586. doi: 10.1038/srep42586. Sci Rep. 2017. PMID: 28195208 Free PMC article. - FMRFa receptor stimulated Ca2+ signals alter the activity of flight modulating central dopaminergic neurons in Drosophila melanogaster.
Ravi P, Trivedi D, Hasan G. Ravi P, et al. PLoS Genet. 2018 Aug 15;14(8):e1007459. doi: 10.1371/journal.pgen.1007459. eCollection 2018 Aug. PLoS Genet. 2018. PMID: 30110323 Free PMC article.
References
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS (2002) Wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila Neuron 33: 545-558. - PubMed
- Acharya JK, Jalink K, Hardy RW, Hartenstein V, Zuker CS (1997) InsP3 receptor is essential for growth and differentiation but not for vision in Drosophila Neuron 18: 881-887. - PubMed
- Baier A, Wittek B, Brembs B (2002) Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol 205: 1233-1240. - PubMed
- Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U (2000) Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila Curr Biol 10: 187-194. - PubMed
- Benzer S (1973) Genetic dissection of behavior. Sci Am 229: 24-37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases