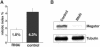Megator, an essential coiled-coil protein that localizes to the putative spindle matrix during mitosis in Drosophila - PubMed (original) (raw)
Megator, an essential coiled-coil protein that localizes to the putative spindle matrix during mitosis in Drosophila
Hongying Qi et al. Mol Biol Cell. 2004 Nov.
Abstract
We have used immunocytochemistry and cross-immunoprecipitation analysis to demonstrate that Megator (Bx34 antigen), a Tpr ortholog in Drosophila with an extended coiled-coil domain, colocalizes with the putative spindle matrix proteins Skeletor and Chromator during mitosis. Analysis of P-element mutations in the Megator locus showed that Megator is an essential protein. During interphase Megator is localized to the nuclear rim and occupies the intranuclear space surrounding the chromosomes. However, during mitosis Megator reorganizes and aligns together with Skeletor and Chromator into a fusiform spindle structure. The Megator metaphase spindle persists in the absence of microtubule spindles, strongly implying that the existence of the Megator-defined spindle does not require polymerized microtubules. Deletion construct analysis in S2 cells indicates that the COOH-terminal part of Megator without the coiled-coil region was sufficient for both nuclear as well as spindle localization. In contrast, the NH2-terminal coiled-coil region remains in the cytoplasm; however, we show that it is capable of assembling into spherical structures. On the basis of these findings we propose that the COOH-terminal domain of Megator functions as a targeting and localization domain, whereas the NH2-terminal domain is responsible for forming polymers that may serve as a structural basis for the putative spindle matrix complex.
Figures
Figure 1.
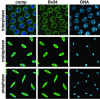
Syncytial Drosophila embryo nuclei labeled by mAb Bx34 and Hoechst from various stages of the cell cycle (inter-, meta-, and anaphase). The labeling by mAb Bx34 is shown in green and the labeling of DNA by Hoechst in blue. The composite (comp) images of the stainings are to the left. At interphase the Bx34 antibody labels the nuclear rim together with interior nuclear labeling. At meta- and anaphase the Bx34 antibody labels a spindle-like structure. All images in these panels are from confocal sections.
Figure 2.
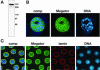
Immunoblot and interphase nuclear labeling of the Megator mAb 12F10. (A) Western blot analysis of Drosophila embryonic protein extract shows that mAb 12F10 recognizes Megator protein as a 260-kDa band. The migration of molecular-weight markers are indicated to the right in black numerals. (B) Larval polytene nucleus labeled with mAb 12F10 (green) and Hoechst (blue). The composite image (comp) clearly indicates that the Megator labeling by mAb 12F10 surrounds the chromosomal DNA labeled by Hoechst. (C) Triple labelings using mAb 12F10 to visualize Megator (green), antilamin antibody to visualize the nuclear lamina (red), and Hoechst to visualize the DNA (blue) of interphase syncytial embryonic nuclei. The composite image (comp) shows that Megator and lamin labeling overlaps at the nuclear rim (yellow color), whereas interior nuclear Megator is interspersed with the DNA labeling of Hoechst. The images are from confocal sections.
Figure 3.
The dynamic redistribution of Megator relative to the putative spindle matrix protein Skeletor during the cell cycle. The images are from double labelings of Megator with mAb 12F10 (green) and Skeletor with mAb 1A1 (red). The composite images (comp) are shown to the left. (A) At interphase Skeletor and Megator labeling are intermingled in the nuclear interior, whereas Megator labeling is prominent at the nuclear rim. During prometa- and anaphase the composite images (comp) show extensive overlap between Megator and Skeletor labeling as indicated by the predominantly yellow color. At telophase where Skeletor begins to redistribute back to the chromosomes, Megator appears to be preferentially localized to the spindle midbody. The images are from confocal sections of syncytial embryonic nuclei. (B) Light squash of a larval polytene nucleus, where Skeletor localized on the chromosomes are surrounded by Megator labeling.
Figure 4.
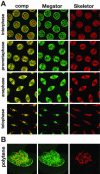
Nuclei from cold- or nocodazole-treated embryos at metaphase. (A) Control (top panel) and cold-treated (bottom panel) embryos triple-labeled with mAb 12F10 (green), rat α-tubulin antibody (red), and Hoechst (DNA in blue). In the cold-treated embryo microtubule spindles have completely depolymerized, as indicated by the absence of microtubule labeling. The mAb 12F10 labeled spindle (green) is still intact, demonstrating that this structure persists independently of the microtubule spindle. (B) Triple-labeling with mAb 12F10 (Megator in green), mAb 1A1 (Skeletor in red), and Hoechst (DNA in blue) from an embryo where microtubules were depolymerized with nocodazole. Both Megator and Skeletor labeling are still present and show extensive colocalization (yellow color in the composite [comp] image). All images are from confocal sections.
Figure 5.
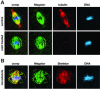
Nuclear localization of Megator in S2 cells. (A) Interphase nucleus labeled with mAb 12F10 (Megator in green), lamin antibody (red), and Hoechst (DNA in blue). The composite image (Megator/lamin) shows considerable overlap (yellow color) between Megator and lamin at the nuclear rim, whereas only Megator is detected in the nuclear interior. (B) Metaphase cell labeled with mAb 12F10 (Megator in green), mAb 1A1 (Skeletor in red), and Hoechst (DNA in blue). Megator and Skeletor labeling show extensive overlap (yellow color in the composite image [comp]) at the Skeletor defined spindle. All images are from confocal sections.
Figure 6.
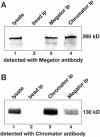
Megator and Chromator immunoprecipitation assays. (A) Immunoprecipitation (ip) of lysates from Drosophila embryos were performed using Chromator antibody (mAb 12H9, lane 4) and Megator antibody (mAb 12F10, lane 3) coupled to immunobeads or with immunobeads only as a control (lane 2). The immunoprecipitations were analyzed by SDS-PAGE and Western blotting using Megator mAb 12F10 for detection. Megator antibody staining of embryo lysate is shown in lane 1. Megator is detected in the Megator and Chromator immunoprecipitation samples as a 260-kDa band (lane 3 and 4, respectively) but not in the control sample (lane 2). (B) Immunoprecipitation (ip) of lysates from Drosophila embryos were performed using Chromator antibody (mAb 12H9, lane 3) and Megator antibody (mAb 12F10, lane 4) coupled to immunobeads or with immunobeads only as a control (lane 2). The immunoprecipitations were analyzed by SDS-PAGE and Western blotting using Chromator mAb 6H11 for detection. Chromator antibody staining of embryo lysate is shown in lane 1. Chromator is detected in the Megator and Chromator immunoprecipitation samples as a 260-kDa band (lanes 4 and 3, respectively) but not in the control sample (lane 2).
Figure 7.
P-element insertion in the Megator gene. (A) Diagram of the Megator genomic locus. The locus has five exons separated by four introns. The P-element insertion site of line l(2)k03905 at the +1 position of the Megator cDNA is indicated by the triangle. The ORF coding for the Megator protein including the position of the coiled-coil region and predicted nuclear localization signal (NLS) is depicted underneath. (B) Megator protein expression in homozygous l(2)k03905 mutant embryos from l(2)k03905/CyO parents. The level of Megator expression in l(2)k03905/CyO and CyO/CyO embryos from the same cross served as a control. The immunoblots were labeled with the anti-Megator Bx34 antibody and with antitubulin antibody. Protein extracts from 35 15–20-h embryos per lane were separated by SDS-PAGE. The relative level of Megator protein expression in mutant embryos as a percentage of Megator expression in control embryos is shown to the right.
Figure 8.
RNAi depletion of Megator in S2 cells leads to a reduction of cells undergoing mitosis. (A) Comparison of the mitotic index of Megator RNAi treated (n = 5) and control (n = 5) S2 cell cultures. The mitotic index was defined as the number of cells in meta- and anaphase as a percentage of total cell number. Megator RNAi treated S2 cell cultures had nearly 60% fewer cells undergoing cell division than mock-treated control cultures. This difference is statistically significant on the p < 0.0025 level (Student's t test). (B) Western blot with Megator antibody of control-treated and Megator RNAi-treated S2 cells. In the RNAi sample Megator protein level is reduced to ∼8% of the level observed in the control cells. Tubulin levels are shown as a loading control.
Figure 9.
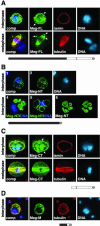
Expression of V5-tagged Megator deletion constructs in S2 cells. The expressed constructs are diagrammed beneath the micrographs. (A) Full-length V5-tagged Megator (Meg-FL) localizes to the nuclear interior and nuclear rim (arrows) of S2 cells at interphase (top panel). The cells were triple-labeled with V5-antibody to visualize the Meg-FL construct (green), lamin antibody (red), and Hoechst to visualize the DNA (blue). The bottom panel shows S2 cells at metaphase labeled with V5-antibody (green), tubulin antibody (red), and Hoechst (DNA in blue). As shown in the composite image (comp) Meg-FL labeling overlaps that of tubulin (yellow color). However, the overexpressed Meg-FL construct also show some aggregation (white arrows). (B) V5-tagged NH2-terminal Megator deletion construct (Meg-NT) truncated just before the end of the coiled-coil region localizes to the cytoplasm and is mainly absent from the nucleus (top panel). The Meg-NT construct was visualized with V5-antibody (green) and the DNA with Hoechst (blue). In 30% of S2 cells the Meg-NT construct formed several large spheres outside the nucleus (bottom panel). (B4) Maximum projection image from a Meg-NT (green) transfected S2 cell double-labeled with Hoechst (blue). (B5) Single confocal section through a transfected S2 cell demonstrating that the spheres are hollow. (B6) Stereo image of a Meg-NT–transfected cell illustrating the spatial relationship between the spheres. (C) S2 cells at inter- and metaphase expressing a V5-tagged COOH-terminal deletion construct (Meg-CT) lacking the coiled-coil domain. At interphase Meg-CT localizes to the nuclear interior and to the nuclear rim (white arrows). The nucleus was labeled with V5-antibody (green), lamin antibody (red), and the DNA with Hoechst (blue). At metaphase (bottom panel) Meg-CT colocalizes with the microtubule spindle as indicated by the yellow color in the composite image (comp). The cell was labeled with V5-antibody (green), tubulin antibody (red), and the DNA with Hoechst (blue). (D) Interphase labeling in the cytoplasm of an S2 cell expressing the Meg-M construct. The cell was labeled with V5-antibody (green), tubulin antibody (red), and the DNA with Hoechst (blue). All images are from confocal sections. On the diagrams the coiled-coil region is in black, the NLS is indicated by a black bar, and the V5-tag by a gray circle.
Similar articles
- EAST interacts with Megator and localizes to the putative spindle matrix during mitosis in Drosophila.
Qi H, Rath U, Ding Y, Ji Y, Blacketer MJ, Girton J, Johansen J, Johansen KM. Qi H, et al. J Cell Biochem. 2005 Aug 15;95(6):1284-91. doi: 10.1002/jcb.20495. J Cell Biochem. 2005. PMID: 15962301 - Chromator, a novel and essential chromodomain protein interacts directly with the putative spindle matrix protein skeletor.
Rath U, Wang D, Ding Y, Xu YZ, Qi H, Blacketer MJ, Girton J, Johansen J, Johansen KM. Rath U, et al. J Cell Biochem. 2004 Nov 15;93(5):1033-47. doi: 10.1002/jcb.20243. J Cell Biochem. 2004. PMID: 15389869 - Skeletor, a novel chromosomal protein that redistributes during mitosis provides evidence for the formation of a spindle matrix.
Walker DL, Wang D, Jin Y, Rath U, Wang Y, Johansen J, Johansen KM. Walker DL, et al. J Cell Biol. 2000 Dec 25;151(7):1401-12. doi: 10.1083/jcb.151.7.1401. J Cell Biol. 2000. PMID: 11134070 Free PMC article. - The spindle matrix through the cell cycle in Drosophila.
Johansen J, Johansen KM. Johansen J, et al. Fly (Austin). 2009 Jul-Sep;3(3):213-20. Epub 2009 Jul 23. Fly (Austin). 2009. PMID: 19690461 Review. - Mitosis, microtubules, and the matrix.
Scholey JM, Rogers GC, Sharp DJ. Scholey JM, et al. J Cell Biol. 2001 Jul 23;154(2):261-6. doi: 10.1083/jcb.200101097. J Cell Biol. 2001. PMID: 11470815 Free PMC article. Review.
Cited by
- dKDM2 couples histone H2A ubiquitylation to histone H3 demethylation during Polycomb group silencing.
Lagarou A, Mohd-Sarip A, Moshkin YM, Chalkley GE, Bezstarosti K, Demmers JA, Verrijzer CP. Lagarou A, et al. Genes Dev. 2008 Oct 15;22(20):2799-810. doi: 10.1101/gad.484208. Genes Dev. 2008. PMID: 18923078 Free PMC article. - To the pore and through the pore: a story of mRNA export kinetics.
Oeffinger M, Zenklusen D. Oeffinger M, et al. Biochim Biophys Acta. 2012 Jun;1819(6):494-506. doi: 10.1016/j.bbagrm.2012.02.011. Epub 2012 Feb 22. Biochim Biophys Acta. 2012. PMID: 22387213 Free PMC article. Review. - Biophysics of mitosis.
McIntosh JR, Molodtsov MI, Ataullakhanov FI. McIntosh JR, et al. Q Rev Biophys. 2012 May;45(2):147-207. doi: 10.1017/S0033583512000017. Epub 2012 Feb 10. Q Rev Biophys. 2012. PMID: 22321376 Free PMC article. Review. - Early spindle assembly in Drosophila embryos: role of a force balance involving cytoskeletal dynamics and nuclear mechanics.
Cytrynbaum EN, Sommi P, Brust-Mascher I, Scholey JM, Mogilner A. Cytrynbaum EN, et al. Mol Biol Cell. 2005 Oct;16(10):4967-81. doi: 10.1091/mbc.e05-02-0154. Epub 2005 Aug 3. Mol Biol Cell. 2005. PMID: 16079179 Free PMC article. - Evidence for a role of spindle matrix formation in cell cycle progression by antibody perturbation.
Yao C, Wang C, Li Y, Zavortink M, Archambault V, Girton J, Johansen KM, Johansen J. Yao C, et al. PLoS One. 2018 Nov 28;13(11):e0208022. doi: 10.1371/journal.pone.0208022. eCollection 2018. PLoS One. 2018. PMID: 30485354 Free PMC article.
References
- Bellotto, M., Bopp, D., Senti, K.-A., Burke, R., Deak, P., Maroy, P., Dickson, B., Basler, K., and Hafen, E. (2002). Maternal-effect loci involved in Drosophila oogenesis and embryogenesis: P element-induced mutations on the third chromosome. Int. J. Dev. Biol. 46, 149-157. - PubMed
- Frasch, M., Glover, D.M., and Saumweber, H. (1986). Nuclear antigens follow different pathways into daughter nuclei during mitosis in early Drosophila embryos. J. Cell Sci. 82, 155-172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous