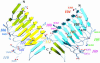A high-affinity interaction with ADP-actin monomers underlies the mechanism and in vivo function of Srv2/cyclase-associated protein - PubMed (original) (raw)
A high-affinity interaction with ADP-actin monomers underlies the mechanism and in vivo function of Srv2/cyclase-associated protein
Pieta K Mattila et al. Mol Biol Cell. 2004 Nov.
Abstract
Cyclase-associated protein (CAP), also called Srv2 in Saccharomyces cerevisiae, is a conserved actin monomer-binding protein that promotes cofilin-dependent actin turnover in vitro and in vivo. However, little is known about the mechanism underlying this function. Here, we show that S. cerevisiae CAP binds with strong preference to ADP-G-actin (Kd 0.02 microM) compared with ATP-G-actin (Kd 1.9 microM) and competes directly with cofilin for binding ADP-G-actin. Further, CAP blocks actin monomer addition specifically to barbed ends of filaments, in contrast to profilin, which blocks monomer addition to pointed ends of filaments. The actin-binding domain of CAP is more extensive than previously suggested and includes a recently solved beta-sheet structure in the C-terminus of CAP and adjacent sequences. Using site-directed mutagenesis, we define evolutionarily conserved residues that mediate binding to ADP-G-actin and demonstrate that these activities are required for CAP function in vivo in directing actin organization and polarized cell growth. Together, our data suggest that in vivo CAP competes with cofilin for binding ADP-actin monomers, allows rapid nucleotide exchange to occur on actin, and then because of its 100-fold weaker binding affinity for ATP-actin compared with ADP-actin, allows other cellular factors such as profilin to take the handoff of ATP-actin and facilitate barbed end assembly.
Figures
Figure 1.
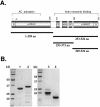
Purified fragments of Srv2/CAP. (A) A schematic representation of Srv2/CAP domains: AC, adenylyl cyclase binding domain; PP, proline-rich motifs (for exact sequences, see Figure 5); WH2, WASp-homology domain 2; D, sites that mediate Srv2/CAP dimerization or multimerization (Huberstey et al., 1996; Zelicof et al., 1996; Yu et al., 1999). The second proline-rich domain binds to the SH3 domain of Abp1 and is required for proper localization of Srv2 in vivo (Lila and Drubin, 1997). The fragments of Srv2/CAP purified and assayed for actin-binding in this study are indicated below by solid lines. (B) Coomassie-stained SDS gel showing the purified Srv2/CAP fragments: lane 1, Srv21-259; lane 2, Srv2253-526; lane 3, Srv2369-526; and lane 4, Srv2253-373.
Figure 2.
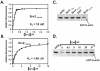
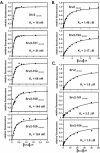
Srv2253-526 binds ADP-actin monomers with high affinity and competes with cofilin for binding actin. (A and B) The increase in the fluorescence of 0.2 μM NBD-labeled MgADP-G-actin was measured under physiological ionic conditions at pH 8.0 over a range of concentrations of Srv2253-526 (A) and Srv2369-526 (B). Symbols are data, and lines are the calculated binding curves for a complex with 1:1 stoichiometry. Dissociation constants (_K_d) were calculated from the binding curves in the figures as described in Materials and Methods. (C) Srv2253-526 depletes significant amounts of ADP-actin from the supernatant in a pull-down assay, whereas Srv2253-373 and Srv21-259 fragments or GST alone do not. The concentrations of actin and GST-fusion proteins in this assay were 3 and 9 μM, respectively. The leftmost lane shows the amount of actin in the supernatant when the control assay was carried out without glutathione beads. (D) Yeast cofilin and Srv2253-526 compete with each other in actin binding. The ADP-actin concentration in the supernatant depletion pull-down assay was 3 μM, and the concentration of GST-Srv2253-526 was 9 μM. Addition of soluble yeast cofilin (4.5-37 μM) decreases the amount of actin monomers sequestered by GST-Srv2253-526. The leftmost lane shows the amount of actin in the supernatant when the assay was carried out in the absence of cofilin and GST-Srv2253-526.
Figure 3.
Srv2253-526 binds ATP-actin monomers with only a modest affinity. (A) Competition for ATP-actin binding between MIM-CT and Srv2253-526. A range of Srv2253-526 concentrations were added to reactions containing 0.2 μM ATP-actin and 0.3 μM MIM-CT at PH 8.0, and the change in fluorescence was measured. Symbols are data, and the solid lines are fitted binding curves for a complex with 1:1 stoichiometry. (B) Determination of the actin nucleotide state binding preference of Srv2253-526 by actin depletion pull-down assay. Reactions contained 3 μM ADP-actin, 2.3 μM ATP-actin, and 9 μM GSTSrv2253-526. As controls, the levels of actin in the supernatants are shown for reactions containing GST alone or lacking glutathione-agarose beads.
Figure 4.
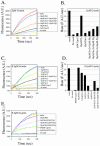
Srv2 inhibits actin monomer addition to barbed ends of filaments. (A) Monomeric actin (3 μM; 10% pyrene-labeled) was preincubated with profilin and/or Srv2253-526 (final concentrations indicated) and added to mechanically sheared actin filament seeds (333 nM) to assay elongation at barbed and pointed ends. (B) Rates of filament elongation from A were calculated as in Moseley et al. (2003). (C) Monomeric actin (0.5 μM; 10% pyrene-labeled) was preincubated with profilin and/or Srv2253-526 and added to F-actin seeds as in A to initiate elongation specifically at the barbed ends of filaments. (D) Rates of filament elongation from C. (E) The same as C, but testing effects of Srv2369-526 on elongation at barbed ends of filaments.
Figure 9.
Structure and stability of wild-type and mutant Srv2 proteins. (A) The folding of wild-type and mutant Srv2253-526 proteins was compared by measuring the far UV CD spectra. The spectra of wild-type Srv2253-526 (solid line), Srv2-104253-526 (dashed line, “longest dashes”), Srv2-108253-526 (dashed line, “medium dashes”), and Srv2-109253-526 (dashed line, “shortest dashes”) are almost identical, suggesting similar structures. (B) The stability of wild-type Srv2253-526 and Srv2369-526 proteins, and mutant Srv2253-526 proteins defective in actin binding, were measured by fluorescence-monitored urea denaturation assay. The normalized fluorescence is shown on the _y_-axis and the urea concentration on the _x_-axis. Both wild-type Srv2 fragments unfold at ∼4 M urea. The mutants unfold at a slightly lower urea concentration, 2.5-3.5 M.
Figure 5.
Alignment of CAP sequences from multiple species. Amino acid sequences from the C-terminal halves of mouse CAP1 and CAP2, Drosophila melanogaster CAP, Arabidopsis thaliana CAP, and S. cerevisiae Srv2/CAP were aligned using the Clustal X program. Regions encompassed by the Srv2253-526 and Srv2369-526 constructs are indicated by lines above the alignment, and the position of WH2 domain is indicated by a dashed line. The residues replaced by alanine in each srv2 allele (101-112) generated in this study (see Table 1) are indicated below the aligned sequences.
Figure 6.
Structural positions of mutated residues in srv2 alleles. The ribbon structure of the dimeric C-terminus (amino acids 369-526) of S. cerevisiae Srv2 is shown (PDB code 1K4Z, details of the structure in Dodatko et al., 2004); the two Srv2 monomers are colored yellow and aqua. Residues altered in each allele are color coded, and each allele is highlighted only once on the twofold symmetric dimer. Each labeled residue was altered to alanine in the relevant allele (Table 1). Srv2-101 and Srv2-102 are not shown, because the residues altered in these two alleles reside outside of the structurally solved domain.
Figure 7.
Growth phenotypes of yeast cells expressing wild-type and mutant alleles of the SRV2 gene. An srv2 null strain of S. cerevisiae (BGY330) was transformed with low copy plasmids expressing wild-type or mutant srv2 alleles under the control of the SRV2 promoter. Transformed cells were grown to saturation, plated in 10-fold serial dilutions, and grown at 25 or 37°C.
Figure 8.
Actin organization defects in cells expressing specific srv2 mutant alleles. _srv2_Δ cells were transformed with low copy plasmids expressing wild-type SRV2, vector alone, or specific srv2 alleles (srv2-104, srv2-108, and srv2-10) under the control of the SRV2 promoter. Cells were grown to log phase at 25°C, fixed, and stained with rhodamine phalloidin.
Figure 10.
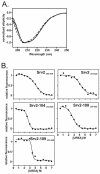
Srv2 alleles that show defects in vivo weaken binding of Srv2 to ADP-actin in vitro. (A) The same assay as described in Figure 2 was used to measure binding affinities of mutant Srv2 fragments for ADP-G-actin. (B) The Srv2-104 mutation impairs binding to ADP-G-actin in Srv2-104369-526. (C) The same assay as in Figure 3A was used to measure the binding affinities of Srv2253-526 mutants for ATP-G-actin. All data are summarized in Table 3.
Similar articles
- Mechanism and biological role of profilin-Srv2/CAP interaction.
Bertling E, Quintero-Monzon O, Mattila PK, Goode BL, Lappalainen P. Bertling E, et al. J Cell Sci. 2007 Apr 1;120(Pt 7):1225-34. doi: 10.1242/jcs.000158. J Cell Sci. 2007. PMID: 17376963 - Reconstitution and dissection of the 600-kDa Srv2/CAP complex: roles for oligomerization and cofilin-actin binding in driving actin turnover.
Quintero-Monzon O, Jonasson EM, Bertling E, Talarico L, Chaudhry F, Sihvo M, Lappalainen P, Goode BL. Quintero-Monzon O, et al. J Biol Chem. 2009 Apr 17;284(16):10923-34. doi: 10.1074/jbc.M808760200. Epub 2009 Feb 6. J Biol Chem. 2009. PMID: 19201756 Free PMC article. - Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1.
Balcer HI, Goodman AL, Rodal AA, Smith E, Kugler J, Heuser JE, Goode BL. Balcer HI, et al. Curr Biol. 2003 Dec 16;13(24):2159-69. doi: 10.1016/j.cub.2003.11.051. Curr Biol. 2003. PMID: 14680631 - WH2 domain: a small, versatile adapter for actin monomers.
Paunola E, Mattila PK, Lappalainen P. Paunola E, et al. FEBS Lett. 2002 Feb 20;513(1):92-7. doi: 10.1016/s0014-5793(01)03242-2. FEBS Lett. 2002. PMID: 11911886 Review. - Regulation of cytoskeletal dynamics by actin-monomer-binding proteins.
Paavilainen VO, Bertling E, Falck S, Lappalainen P. Paavilainen VO, et al. Trends Cell Biol. 2004 Jul;14(7):386-94. doi: 10.1016/j.tcb.2004.05.002. Trends Cell Biol. 2004. PMID: 15246432 Review.
Cited by
- Cyclase-associated protein (CAP) acts directly on F-actin to accelerate cofilin-mediated actin severing across the range of physiological pH.
Normoyle KPM, Brieher WM. Normoyle KPM, et al. J Biol Chem. 2012 Oct 12;287(42):35722-35732. doi: 10.1074/jbc.M112.396051. Epub 2012 Aug 17. J Biol Chem. 2012. PMID: 22904322 Free PMC article. - Structure and function of a G-actin sequestering protein with a vital role in malaria oocyst development inside the mosquito vector.
Hliscs M, Sattler JM, Tempel W, Artz JD, Dong A, Hui R, Matuschewski K, Schüler H. Hliscs M, et al. J Biol Chem. 2010 Apr 9;285(15):11572-83. doi: 10.1074/jbc.M109.054916. Epub 2010 Jan 18. J Biol Chem. 2010. PMID: 20083609 Free PMC article. - Mammalian CAP (Cyclase-associated protein) in the world of cell migration: Roles in actin filament dynamics and beyond.
Zhou GL, Zhang H, Field J. Zhou GL, et al. Cell Adh Migr. 2014;8(1):55-9. doi: 10.4161/cam.27479. Epub 2013 Jan 1. Cell Adh Migr. 2014. PMID: 24429384 Free PMC article. - Cytosolic concentrations of actin binding proteins and the implications for in vivo F-actin turnover.
Gonzalez Rodriguez S, Wirshing ACE, Goodman AL, Goode BL. Gonzalez Rodriguez S, et al. J Cell Biol. 2023 Dec 4;222(12):e202306036. doi: 10.1083/jcb.202306036. Epub 2023 Oct 6. J Cell Biol. 2023. PMID: 37801069 Free PMC article. - Srv2 Is a Pro-fission Factor that Modulates Yeast Mitochondrial Morphology and Respiration by Regulating Actin Assembly.
Chen YC, Cheng TH, Lin WL, Chen CL, Yang WY, Blackstone C, Chang CR. Chen YC, et al. iScience. 2019 Jan 25;11:305-317. doi: 10.1016/j.isci.2018.12.021. Epub 2018 Dec 26. iScience. 2019. PMID: 30639852 Free PMC article.
References
- Balcer, H.I., Goodman, A.L., Rodal, A.A., Smith, E., Kugler, J., Heuser, J.E., and Goode, B.L. (2003). Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr. Biol. 13, 2159-2169. - PubMed
- Baum, B., Li, W., and Perrimon, N. (2000). A cyclase-associated protein regulates actin and cell polarity during Drosophila oogenesis and in yeast. Curr. Biol. 10, 964-973. - PubMed
- Benlali, A., Draskovic, I., Hazelett, D.J., and Treisman, J.E. (2000). act up controls actin polymerization to alter cell shape and restrict Hedgehog signaling in the Drosophila eye disc. Cell 101, 271-281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous