Null mutation of alpha1D Ca2+ channel gene results in deafness but no vestibular defect in mice - PubMed (original) (raw)
Null mutation of alpha1D Ca2+ channel gene results in deafness but no vestibular defect in mice
Hongwei Dou et al. J Assoc Res Otolaryngol. 2004 Jun.
Abstract
Multiple Ca2+ channels confer diverse functions to hair cells of the auditory and vestibular organs in the mammalian inner ear. We used gene-targeting technology to generate alpha1D Ca2+ channel-deficient mice to determine the physiological role of these Ca2+ channels in hearing and balance. Analyses of auditory-evoked brainstem recordings confirmed that alpha1D-/- mice were deaf and revealed that heterozygous (alpha1D+/-) mice have increased hearing thresholds. However, hearing deficits in alpha1D+/- mice were manifested mainly by the increase in threshold of low-frequency sounds. In contrast to impaired hearing, alpha1D-/- mice have balance performances equivalent to their wild-type littermates. Light and electron microscope analyses of the inner ear revealed outer hair cell loss at the apical cochlea, but no apparent abnormality at the basal cochlea and the vestibule. We determined the mechanisms underlying the auditory function defects and the normal vestibular functions by examining the Ba2+ currents in cochlear inner and outer hair cells versus utricular hair cells in alpha1D+/- mice. Whereas the whole-cell Ba2+ currents in inner hair cells consist mainly of the nimodipine-sensitive current (approximately 85%), the utricular hair cells express only approximately 50% of this channel subtype. Thus, differential expression of alpha1D channels in the cochlear and utricular hair cells confers the phenotype of the alpha1D null mutant mice. Because vestibular and cochlear hair cells share common features and null deletion of several genes have yielded both deafness and imbalance in mice, alpha1D null mutant mice may serve as a model to disentangle vestibular from auditory-specific functions.
Figures
Figure 1
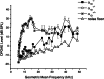
ABR thresholds from the right ears of wild-type (A), heterozygous (B), and homozygous (C) mice (5–8 week old). The sound pressure levels in dB of broadband clicks (0.1 ms) delivered to the ear are indicated on the left side of the traces. Representative normal broadband click responses from α1D+/+ mice are shown; the heterozygous mice α1D+/− showed increased threshold. No response was observed from any of the 19 α1D−/− mice examined. D. ABR thresholds for α1D+/+ (n = 20) and α1D+/−(n = 28) mice in response to broadband clicks and 3-ms pure tones of 8, 16, and 32 kHz (p < 0.05 at 8 and 16 kHz and p = 0.3 at 32 kHz). The asterisks denote the comparisons, which are statistically significant. The data are means ± SD.
Figure 2
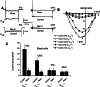
α1D Ca2+ channel-deficient mice had normal swimming performance and no difficulty maintaining their balance. A,B. α1D−/− mice are shown performing a swim test in a 37°C water bath and balancing on a stationary cylinder. C. The times taken to swim to a target (a dry platform) and to remain on the stationary and rotating cylinder (5 rpm) are illustrated in the histogram. Seven animals from each genotype were tested. The mean of 10 trials for each animal is reported. The mean values for the swim test were as follows (in seconds): α1D+/+, = 130 ± 13; α1D−/−, X̄ = 132 ± 15 (n = 7) p = 0.3, NS, and the means values for the balance test were (in seconds): α1D+/+, X̄ = 85 ± 8; α1D−/−, X̄ = 87 + 8 (n = 7) p = 0.3, NS. To eliminate fatigue factor, each trial was separated by a 10-min rest period. No reward was given to the animals during or after the test.
Figure 3
Scanning electron micrographs of apical (A,B) and basal (C,D) cochleae of α1D+/+ and α1D−/− mice showing robust loss of hair bundles at the apical turn of the mutant cochlea. The basal turn OHCs of α1D−/− mice appear normal (D) and are indistinguishable from the wild-type (C). In contrast to the morphology of the apical turn of the mutant cochlea in which OHCs are devoid of hair bundles, the saccule (E,F) and utricle (G,H) of the mutant mice have intact hair bundles. Scale bar: A–H, 10 μm.
Figure 4
Light micrographs of cross-sections of the cochlear duct from 6-week-old α1D+/+ (A– C) and α1D−/− (D – F) mice. Reissner’s membrane (RM) separating the scala media (SM) and scala vestibuli (SV) in both the wild-type and the mutant cochlea remain intact (A– F). Normal histology of the wild-type mouse shows apex (A) and base (C) for comparison. IHCs and OHCs are identifiable at the apex and base of the cochlea in α1D+/+ (A,C). Shown in B are densely packed cells in the spiral ganglion. Although the cell bodies of OHCs at the apex can be seen in the α1D−/−, the hair bundles were absent in the serial sections of the cochlea. More severely affected regions at the apex show fewer identifiable OHCs. However, the IHCs at the apex remain relatively intact in the mutant (D). OHCs and IHCs at the base of wild-type and mutant cochlea were normal. Schwann cells and myelinated nerve fibers that fill Rosenthal’s canal (R) in the modiolus remain intact in both α1D+/+ and α1D−/− cochlea. Spiral ganglion (G) neuronal cell bodies were reduced in number in some mutants (E,F). Analysis of random cell counts shows that neuronal cell bodies in Gwere 20% less in α1D−/− compared with α1D+/+. Otherwise, the typical architecture of the organ of Corti remains intact in both the wild-type and mutant mice. L, S, SM, ST, SV, T, DC, and TM respectively denote limbus, stria vascularis, scala media, scala tympanic, scala vestibuli, tunnel of Corti, Deiters’ cells, and tympanic membrane. Scale bar: 90 μm inA, 75 μm in B – F.
Figure 5
In the saccule (A,D) and utricle (B,E) of the α1D+/+ and α1D−/− mice, the otoconia (O) are embedded in the otolithic membrane (OM) overlying the neuroepithelium of the macula (M). Tufts of hair bundles and corresponding cell bodies are identifiable in the mutant and wild-type saccule and utricle. The structure of the ampulla of the semicircular ducts of α1D+/+ and α1D−/− (C,F, respectively) and the gelatinous cupula (C) overlying the crista ampullaris (CA) sensory epithelium are normal. Scale bars: 50 μm in A – E and 70 μm in F.
Figure 6
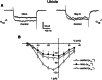
Mean DP-grams for 6 weeks α1D+/+, α1D+/−, and α1D−/− (n = 6) were tested measuring the levels of the 2 _f_1 − _f_2DPOAE over a geometric-mean frequency range from 5.6 to 48.5 kHz, using an _f_2/_f_1of 1.25, and primary tone stimuli at _L_1 =_L_2 = 65 SPL. Clearly, α1D−/− mice yielded no significant DPOAEs at low frequencies (5–15 kHz) compared with α1D+/+ and α1D+/− mice. However, at 20–48 kHz, α1D−/− produced modest DPs.
Figure 7
Inward Ba2+ currents through whole-cell Ca2+ channels recorded from hair cells in intact cochlea. A. Whole-cell Ba2+ currents from IHCs at the apical turn of PI wild-type (α1D+/+) mice were elicited from a holding potential of −60 mV to a step potential of −10 mV. Only current traces that stabilized after initial current rundown were analyzed and shown. After 90 s and an application of nimodipine (20 μM), the whole-cell Ba2+ currents were reduced by ~80% (left upper panel). The right upper panel shows Ba2+ current traces recorded from an OHC at the apical turn of the cochlea and of the effect of nimodipine (20 μM) on the current. For OHCs, nimodipine blocked at least 95% of the whole-cell Ba2+ currents. The middle panels consist of Ba2+ current traces generated from hair cells at the apical cochlear of PI mutant (α1D−/−) mice. In contrast to IHCs (left), OHCs (right) in the α1D−/−did not express Ba2+-permeable inward current channels. B. Current–voltage relationships of Ba2+ currents in hair cells at the apical turn of the cochlea (IHC, n = 9; OHC,n = 7). C. In contrast to OHCs at the apical turn, there were substantial residual currents following the application of nimodipine (20 μM) on OHCs at the basal turn of the cochlea. Group data of Ba2+ current densities shown in a bar graph from IHCs (n = 6) and OHCs (n = 7) at the basal turn of the cochlea in α1D+/+ and α1D−/−mice.
Figure 8
Inward Ba2+ currents through whole-cell Ca2+ channels recorded from hair cells in intact utricle. A. Approximately 50% of the whole-cell Ba2+currents of utricular hair cells from α1D+/+ mice were sensitive to 20 μM nimodipine. As expected, Ba2+ currents of hair cells from the utricle of α1D−/− mice (right panel) were insensitive to Bay K (10 μM). B. Current–voltage relationships of Ba2+ currents from utricular hair cells (n = 9) of α1D+/+ and α1D−/−mice.
Similar articles
- Auditory and vestibular defects in the circling (ci2) rat mutant.
Kaiser A, Fedrowitz M, Ebert U, Zimmermann E, Hedrich HJ, Wedekind D, Löscher W. Kaiser A, et al. Eur J Neurosci. 2001 Oct;14(7):1129-42. doi: 10.1046/j.0953-816x.2001.01726.x. Eur J Neurosci. 2001. PMID: 11683905 - [Mice lacking of voltage-gated L-type calcium channel alpha1D subunit have impaired sinoatrial node function and caused deafness].
Chu H, Zhou X, Song H, Cui Y, Xiong H, Zhou L. Chu H, et al. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007 May;21(10):468-72. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007. PMID: 17650821 Chinese. - Cav1.3 (alpha1D) Ca2+ currents in neonatal outer hair cells of mice.
Michna M, Knirsch M, Hoda JC, Muenkner S, Langer P, Platzer J, Striessnig J, Engel J. Michna M, et al. J Physiol. 2003 Dec 15;553(Pt 3):747-58. doi: 10.1113/jphysiol.2003.053256. Epub 2003 Sep 26. J Physiol. 2003. PMID: 14514878 Free PMC article. - Channeling your inner ear potassium: K(+) channels in vestibular hair cells.
Meredith FL, Rennie KJ. Meredith FL, et al. Hear Res. 2016 Aug;338:40-51. doi: 10.1016/j.heares.2016.01.015. Epub 2016 Feb 4. Hear Res. 2016. PMID: 26836968 Review. - Prestin and the cochlear amplifier.
Dallos P, Zheng J, Cheatham MA. Dallos P, et al. J Physiol. 2006 Oct 1;576(Pt 1):37-42. doi: 10.1113/jphysiol.2006.114652. Epub 2006 Jul 27. J Physiol. 2006. PMID: 16873410 Free PMC article. Review.
Cited by
- Demyelination and Na+ Channel Redistribution Underlie Auditory and Vestibular Dysfunction in PMP22-Null Mice.
Lee JH, Park S, Perez-Flores MC, Chen Y, Kang M, Choi J, Levine L, Gratton MA, Zhao J, Notterpek L, Yamoah EN. Lee JH, et al. eNeuro. 2024 Feb 20;11(2):ENEURO.0462-23.2023. doi: 10.1523/ENEURO.0462-23.2023. Print 2024 Feb. eNeuro. 2024. PMID: 38378628 Free PMC article. - Genetic and pharmacologic alterations of claudin9 levels suffice to induce functional and mature inner hair cells.
Chen Y, Lee JH, Li J, Park S, Perez Flores MC, Peguero B, Kersigo J, Kang M, Choi J, Levine L, Gratton MA, Fritzsch B, Yamoah EN. Chen Y, et al. bioRxiv [Preprint]. 2024 Nov 8:2023.10.08.561387. doi: 10.1101/2023.10.08.561387. bioRxiv. 2024. PMID: 37873357 Free PMC article. Preprint. - mTORC2 regulates auditory hair cell structure and function.
Cortada M, Levano S, Hall MN, Bodmer D. Cortada M, et al. iScience. 2023 Aug 19;26(9):107687. doi: 10.1016/j.isci.2023.107687. eCollection 2023 Sep 15. iScience. 2023. PMID: 37694145 Free PMC article. - Signal transmission in mature mammalian vestibular hair cells.
Spaiardi P, Marcotti W, Masetto S, Johnson SL. Spaiardi P, et al. Front Cell Neurosci. 2022 Jul 22;16:806913. doi: 10.3389/fncel.2022.806913. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35936492 Free PMC article. - Multiple Calcium Channel Types with Unique Expression Patterns Mediate Retinal Signaling at Bipolar Cell Ribbon Synapses.
Zhang G, Liu JB, Yuan HL, Chen SY, Singer JH, Ke JB. Zhang G, et al. J Neurosci. 2022 Aug 24;42(34):6487-6505. doi: 10.1523/JNEUROSCI.0183-22.2022. Epub 2022 Jul 27. J Neurosci. 2022. PMID: 35896423 Free PMC article.
References
- Beutner D, Voet T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. - PubMed
- Ertel AA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez–Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbuamer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. - PubMed
- Flagella M, Clarke LL, Miller ML, Erway LC, Gianella RA, Kramer J, Duffy JJ, Doestchman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na+/K+/2Cl− cotransporter have impaired intestinal chloride secretion and are profoundly deaf. J. Biol. Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous