Highly infectious SARS-CoV pseudotyped virus reveals the cell tropism and its correlation with receptor expression - PubMed (original) (raw)
. 2004 Sep 3;321(4):994-1000.
doi: 10.1016/j.bbrc.2004.07.060.
Peigang Wang, Xuanling Shi, Guangwen Wang, Jian Chen, Aihua Zheng, Wei Wang, Zai Wang, Xiuxia Qu, Min Luo, Lei Tan, Xijun Song, Xiaolei Yin, Jianguo Chen, Mingxiao Ding, Hongkui Deng
Affiliations
- PMID: 15358126
- PMCID: PMC7092805
- DOI: 10.1016/j.bbrc.2004.07.060
Highly infectious SARS-CoV pseudotyped virus reveals the cell tropism and its correlation with receptor expression
Yuchun Nie et al. Biochem Biophys Res Commun. 2004.
Abstract
Studies of SARS coronavirus (SARS-CoV)-the causative agent of severe acute respiratory syndrome (SARS)-have been hampered by its high transmission rate and the pathogenicity of this virus. To permit analysis of the host range and entry mechanism of SARS-CoV, we incorporated the humanized SARS-CoV spike (S) glycoprotein into HIV particles to generate a highly infectious SARS-CoV pseudotyped virus. The infection on Vero E6-a permissive cell line to SARS-CoV-could be neutralized by sera from convalescent SARS patients, and the entry was a pH-dependent process. With these highly infectious SARS-CoV pseudotypes, several cell lines derived from various tissues were revealed as susceptible to SARS-CoV, which were highly corresponding to the expression pattern of virus's receptor angiotensin-converting enzyme 2 (ACE2). In addition, we also demonstrated angiotensin 1 converting enzyme (ACE)-the homologue of ACE2 could not function as a receptor for SARS-CoV.
Figures
Fig. 1
The expression of S protein from a codon-optimized S gene. (A) 293T cells were transfected with pTS, pTSh or pcDNA3.1 (+). The expression of S protein was analyzed by FACS. Transfected cells were stained with sera from SARS patients (hatched shape) or normal sera (open shape). (B) Lysates of transfected 293T cells were analyzed by Western blot using anti-S protein rabbit sera. (C) Codon usage of SARS S glycoprotein (S) and human genes (H). The percentage of each codon in degenerate codons is listed and the most prevalent codon has been shown in bold.
Fig. 2
Generation of HIV/SARS pseudotyped virus. (A) The purified pseudotyped viruses were analyzed by Western blot using anti-S rabbit sera. (B) Several cell lines were infected with the HIV/SARS. The means of luciferase activity of HIV/env- (gray), HIV/TSh (striped), and HIV/VSV-G (black) are shown. (C) Neutralization assays of the HIV/SARS infection: HIV/SARS + patient’s serum (HIV/SARS + PS, black square), HIV/VSV-G + patient’s serum (HIV/VSV-G + PS, black triangle), and HIV/SARS + normal serum (HIV/SARS + NS, white circle). All the data are expressed as percentages of infectivity of control (without sera). (D) Syncytia formation occurred in co-cultured Vero E6 and TSh-COS cells. TS-COS cells and non-transduced COS-7 cells co-cultured with Vero E6 cells were used as controls.
Fig. 3
Analysis of cell tropism and its correlation with the expression pattern of ACE2. (A) Various cell lines were infected with HIV/SARS (black). Pseudotypes with no envelope protein (striped) or with VSV-G (white) were used as negative and positive controls. All infections were performed in triplicate. The error bar stands for SD. (B) Analysis of the ACE2 expression in these cells by RT-PCR. Total RNA from these cells was amplified with primers specific for ACE2 or β-actin as a control (bottom).
Fig. 4
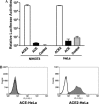
ACE could not function as a receptor for SARS-CoV. The cDNA of ACE or ACE2 was transduced into NIH/3T3 or HeLa cells by retroviral vector pMX. (A) The transduced cells were infected with HIV/SARS pseudovirus and the luciferase activity was assayed 2 days post-infection. Non-transduced NIH3T3 and HeLa cells were used as control. (B) The ACE or ACE2 expressing HeLa cells were incubated with soluble S protein (hatched shape) or control (open shape), anti-S sera, and fluorescence-labeled secondary antibody. The binding of S was analyzed by FACS.
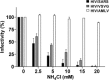
Fig. 5
pH-dependency of HIV/SARS infectivity. Huh7 cells treated with various concentrations of NH4Cl were infected with HIV/SARS. The pH-dependent HIV/VSV-G and pH-independent HIV/AMLV pseudotyped viruses were used as positive and negative controls, respectively.
Similar articles
- Retroviruses pseudotyped with the severe acute respiratory syndrome coronavirus spike protein efficiently infect cells expressing angiotensin-converting enzyme 2.
Moore MJ, Dorfman T, Li W, Wong SK, Li Y, Kuhn JH, Coderre J, Vasilieva N, Han Z, Greenough TC, Farzan M, Choe H. Moore MJ, et al. J Virol. 2004 Oct;78(19):10628-35. doi: 10.1128/JVI.78.19.10628-10635.2004. J Virol. 2004. PMID: 15367630 Free PMC article. - Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus.
Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Li W, et al. Nature. 2003 Nov 27;426(6965):450-4. doi: 10.1038/nature02145. Nature. 2003. PMID: 14647384 Free PMC article. - Vesicular stomatitis virus pseudotyped with severe acute respiratory syndrome coronavirus spike protein.
Fukushi S, Mizutani T, Saijo M, Matsuyama S, Miyajima N, Taguchi F, Itamura S, Kurane I, Morikawa S. Fukushi S, et al. J Gen Virol. 2005 Aug;86(Pt 8):2269-2274. doi: 10.1099/vir.0.80955-0. J Gen Virol. 2005. PMID: 16033974 - Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus.
Kuhn JH, Li W, Choe H, Farzan M. Kuhn JH, et al. Cell Mol Life Sci. 2004 Nov;61(21):2738-43. doi: 10.1007/s00018-004-4242-5. Cell Mol Life Sci. 2004. PMID: 15549175 Free PMC article. Review. - Severe acute respiratory syndrome coronavirus entry as a target of antiviral therapies.
Kuhn JH, Li W, Radoshitzky SR, Choe H, Farzan M. Kuhn JH, et al. Antivir Ther. 2007;12(4 Pt B):639-50. Antivir Ther. 2007. PMID: 17944271 Review.
Cited by
- Tetherin antagonism by SARS-CoV-2 ORF3a and spike protein enhances virus release.
Stewart H, Palmulli R, Johansen KH, McGovern N, Shehata OM, Carnell GW, Jackson HK, Lee JS, Brown JC, Burgoyne T, Heeney JL, Okkenhaug K, Firth AE, Peden AA, Edgar JR. Stewart H, et al. EMBO Rep. 2023 Dec 6;24(12):e57224. doi: 10.15252/embr.202357224. Epub 2023 Oct 11. EMBO Rep. 2023. PMID: 37818801 Free PMC article. - Accelerating antiviral drug discovery: lessons from COVID-19.
von Delft A, Hall MD, Kwong AD, Purcell LA, Saikatendu KS, Schmitz U, Tallarico JA, Lee AA. von Delft A, et al. Nat Rev Drug Discov. 2023 Jul;22(7):585-603. doi: 10.1038/s41573-023-00692-8. Epub 2023 May 12. Nat Rev Drug Discov. 2023. PMID: 37173515 Free PMC article. Review. - Single-Virus Fusion Measurements Reveal Multiple Mechanistically Equivalent Pathways for SARS-CoV-2 Entry.
Sengar A, Cervantes M, Bondalapati ST, Hess T, Kasson PM. Sengar A, et al. J Virol. 2023 May 31;97(5):e0199222. doi: 10.1128/jvi.01992-22. Epub 2023 May 3. J Virol. 2023. PMID: 37133381 Free PMC article. - Biomimetic SARS-CoV-2 Spike Protein Nanoparticles.
Phan A, Avila H, MacKay JA. Phan A, et al. Biomacromolecules. 2023 May 8;24(5):2030-2041. doi: 10.1021/acs.biomac.2c01465. Epub 2023 Mar 31. Biomacromolecules. 2023. PMID: 37001147 - Evaluation of In Vitro and In Vivo Antiviral Activities of Vitamin D for SARS-CoV-2 and Variants.
Mok CK, Ng YL, Ahidjo BA, Aw ZQ, Chen H, Wong YH, Lee RCH, Loe MWC, Liu J, Tan KS, Kaur P, Wang Y, Hao E, Hou X, Tan YW, Deng J, Chu JJH. Mok CK, et al. Pharmaceutics. 2023 Mar 12;15(3):925. doi: 10.3390/pharmaceutics15030925. Pharmaceutics. 2023. PMID: 36986786 Free PMC article.
References
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. - PubMed
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous