Characterization of karyopherin cargoes reveals unique mechanisms of Kap121p-mediated nuclear import - PubMed (original) (raw)
Characterization of karyopherin cargoes reveals unique mechanisms of Kap121p-mediated nuclear import
Deena M Leslie et al. Mol Cell Biol. 2004 Oct.
Erratum in
- Mol Cell Biol. 2004 Nov;24(22):10099
Abstract
In yeast there are at least 14 members of the beta-karyopherin protein family that govern the movement of a diverse set of cargoes between the nucleus and cytoplasm. Knowledge of the cargoes carried by each karyopherin and insight into the mechanisms of transport are fundamental to understanding constitutive and regulated transport and elucidating how they impact normal cellular functions. Here, we have focused on the identification of nuclear import cargoes for the essential yeast beta-karyopherin, Kap121p. Using an overlay blot assay and coimmunopurification studies, we have identified 30 putative Kap121p cargoes. Among these were Nop1p and Sof1p, two essential trans-acting protein factors required at the early stages of ribosome biogenesis. Characterization of the Kap121p-Nop1p and Kap121p-Sof1p interactions demonstrated that, in addition to lysine-rich nuclear localization signals (NLSs), Kap121p recognizes a unique class of signals distinguished by the abundance of arginine and glycine residues and consequently termed rg-NLSs. Kap104p is also known to recognize rg-NLSs, and here we show that it compensates for the loss of Kap121p function. Sof1p is also transported by Kap121p; however, its import can be mediated by a piggyback mechanism with Nop1p bridging the interaction between Sof1p and Kap121p. Together, our data elucidate additional levels of complexity in these nuclear transport pathways.
Figures
FIG. 1.
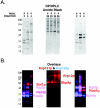
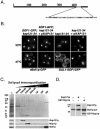
Kap121p interacts with numerous nuclear proteins. (A) Yeast nuclear proteins were purified, HA HPLC fractionated, separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with cytosols from cells synthesizing either Kap121p-pA or Kap123p-pA (see also reference 28). Fractions enriched with Kap121p-pA-interacting proteins were pooled, and proteins were further fractionated by reversed-phase HPLC (RP/HPLC), separated by SDS-PAGE, transferred to nitrocellulose membranes, and detected by amido black staining. (B) Membranes were separately probed with cytosols from cells producing Kap121p-pA or Kap123p-pA, and bound chimeras were detected with horseradish peroxidase-conjugated anti-rabbit IgG and enhanced chemiluminescence. Overlaid, colorized images of each blot (red, Kap121p-pA; blue, Kap123p-pA) are shown. Karyopherin-interacting proteins were identified by MS of protein bands in an identical gel.
FIG. 2.
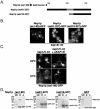
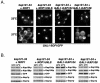
Nop1p mislocalizes in kap121-34 cells. (A) Schematic diagram of Nop1p. The white segment highlights the RG-rich GAR domain of Nop1p. The gray segment represents a basic stretch of amino acids (199 to 219; SHRPGRELISMAKKRPNIIP) that is most similar to the previously identified Kap121p NLS sequences. The NLS sequences of Nab2p and Nab4p (27) were compared to full-length Nop1p by using MegAlign (Lipman-Pearson: ktuple, 2; gap penalty, 4; gap length penalty, 12). (B) The distribution of endogenously tagged NOP1 (_e_Nop1p-GFP) and galactose-inducible NOP1GFP (_GAL1-_Nop1p-GFP) were monitored by direct fluorescence microscopy in kap121-34 cells. NOP1 GFP; kap121-34 cells were visualized after growth to mid-logarithmic phase at the permissive temperature (23°C) and after 3 h of growth at the restrictive temperature (37°C). Although the galactose-inducible NOP1GFP chimera was expressed in kap121-34 cells at 23 or 37°C, _e_Nop1p-GFP remains nucleolar in hap121-34 cells after 3h of growth at 37°C. However, GAL1_-Nop1p-GFP accumulates in the cytoplasm of kap121-34 cells when expressed at the nonpermissive temperature. This contrasts with control kap121-34 cells containing a wild-type copy of KAP121 (kap121-34+p_KAP121) or kap121-34 cells expressing a _GAL1_-cNLS-GFP chimera.
FIG. 3.
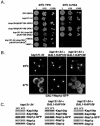
The GAR domain of Nop1p interacts directly with Kap121p and contains a functional NLS. (A) A schematic diagram representing the fragments of Nop1p expressed as GST and GFP fusion proteins. The GAR domain and a putative lysine-rich NLS (aa 199 to 219 [shaded gray]) are indicated. (B) Galactose-inducible GFP fusion proteins containing the three fragments of Nop1p were synthesized at 23°C in kap121-34 cells and analyzed by direct fluorescence microscopy. Note the nuclear localization of Nop1p(aa1-90)-GFP. (C) The cellular localization of Nop1p(aa1-90)-GFP was determined at both 23 and 37°C in kap121-34 and kap121-34+p_KAP121_ cells. Note that Nop1p(aa1-90)-GFP mislocalized to the cytoplasm of in kap121-34 cells at 37°C. (D) GST fusions containing fragments of Nop1p (∼400 ng) or GST alone were immobilized on glutathione-Sepharose 4B beads and incubated with recombinant Kap121p (∼200 ng). Equal amounts of the unbound fractions (UB), bound fractions (B), and the GST fusions alone (A) were analyzed by SDS-PAGE and Coomassie blue staining. Note recombinant Kap121p interacts directly with GST-Nop1p(aa1-90).
FIG. 4.
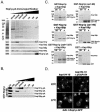
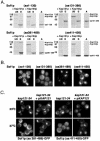
Kap104p can supplant the function of Kap121p in Nop1p import. (A) kap121-34 and kap104::ura3::HIS5 are synthetically lethal in combination. The mutant strains shown were tested for their ability to withstand the loss of a wild-type copy of KAP104 or KAP108 at an otherwise permissive temperature. The indicated dilutions of cultures containing each strain were spotted onto YPD or 5-FOA-containing medium (to select against cells containing p_KAP104-URA3_ or p_KAP108-URA3_) and incubated at 23°C for 4 days. (B) Overexpression of KAP104 rescues the mislocalization of GAL1_-Nop1p-GFP. Kap104p (p_GAL1-KAP104) and Kap108p (p_GAL1-KAP108_) were induced in kap121-34 cells containing GAL1_-Nop1p-GFP (p_GAL1-NOP1GFP) by a shift to galactose-containing medium for 3 h at restrictive (37°C) or permissive (23°C) temperatures. Under these conditions, in the absence of additional Kap104p, Nop1p-GFP accumulates in the cytoplasm but is nuclear upon coinduction of Kap104p. (C) kap121-34, kap121-34+p_GAL1-KAP104_, and kap121-34+p_GAL1-KAP108_ whole-cell lysates from the same cultures as those used for microscopy were probed with rabbit polyclonal α-Kap104p, α-Kap108p, and α-Gsp1p and mouse monoclonal α-Nop1p (MAb D77 [5]) antibodies. The Western blots indicate the relative levels of Kap104p, Kap108p, Nop1p-GFP, endogenous Nop1p, and Gsp1p (loading control).
FIG. 5.
Kap104p interacts directly with Nop1p. (A) Nop1p-pA was immunopurified from NOP1-A cells, and copurifying proteins were eluted with a step gradient of MgCl2 as indicated. Equal volumes of the lysate (L) and elution fractions were separated by SDS-PAGE and either stained with Coomassie brilliant blue (top panel) or transferred to nitrocellulose for immunoblot analysis (bottom panel) with kap-specific polyclonal antibodies as indicated. Nop1p-pA was also detected. The membranes were probed with kap-specific polyclonal antibodies as shown. (B) Recombinant GST-Nop1p(aa1-90) was immobilized on glutathione-Sepharose and incubated with yeast whole-cell lysates (1 mg/ml) from strains expressing Kap121p-pA, Kap104p-pA, Kap123p-pA, and Kap108p-pA. Bound protein complexes were washed and eluted with a step gradient of MgCl2 as indicated. Proteins from each eluate fraction, the unbound fraction (lane UB), and the wash buffer (lane WB) fraction were separated by SDS-PAGE and transferred to nitrocellulose membranes. Protein A chimeras were detected by immunoblotting with horseradish peroxidase-conjugated anti-rabbit IgG and enhanced chemiluminescence. Note that Kap121p preferentially copurified with both Nop1p-pA and GST-Nop1p(aa1-90) and that minimal amounts of Kap104p also copurified with these Nop1p fusion proteins. (C) GST-Nop1p (∼400 ng), GST-Nop1p(aa1-90) (∼400 ng), GST-Nop1(aa91-327) (∼400 ng), and GST alone (∼400 ng) were immobilized on glutathione-Sepharose 4B beads and incubated with recombinant Kap104p (∼200 ng) or Kap108p (∼200 ng) as indicated. Equal amounts of unbound fractions (lanes UB), bound fractions (lanes B), and the GST fusions alone (lanes A) were analyzed by SDS-PAGE and Coomassie blue staining. Kap104p interacted directly with both Nop1p and the N-terminal rg-NLS-containing GAR domain of Nop1p. (D) Nop1p is correctly localized to the nucleolus in kap104 cells. The galactose-inducible NOP1GFP chimera (_GAL1_-Nop1p-GFP) was expressed in kap104-16 cells (in the presence or absence of a wild-type copy of KAP104) at permissive (23°C) or restrictive (37°C) temperatures and visualized by direct fluorescence microscopy. Note that there was no significant change in the localization of Nop1p in these strains at the nonpermissive temperature.
FIG. 6.
Kap121p imports Sof1p. (A) Schematic diagram of Sof1p. The gray segment represents a basic stretch of amino acids that is most similar to the previously identified Kap121p NLS sequences. (B) Endogenously tagged SOF1 GFP (_e_Sof1p-GFP) and galactose-inducible SOF1GFP (_GAL1-_Sof1p-GFP) were monitored by direct fluorescence microscopy in kap121-34 cells at permissive (23°C) and nonpermissive (37°C) temperatures as described previously. Note the cytoplasmic mislocalization of _GAL1_-Sof1p-GFP at 37°C. (C) Sof1p-pA protein complexes were purified from SOF1-A whole-cell lysates. These immobilized complexes were washed extensively, and the bound proteins were eluted with a step gradient of MgCl2 as indicated. Equal volumes of the lysate (lane L) and eluted fractions were separated by SDS-PAGE and either stained with Coomassie brilliant blue (top panel) or transferred to nitrocellulose and analyzed by immunoblotting with rabbit α-Kap121p polyclonal and α-Nop1p monoclonal (MAb D77 [5]) antibodies (bottom panel). Note that both Kap121p and Nop1p copurified with Sof1p-pA. (D) Sof1p-pA was purified from SOF1-A whole-cell lysates, and the copurified proteins were removed by a wash with 1 M MgCl2 (left panel). The beads were incubated with GST-Nop1p or recombinant Kap121p (middle and right panels, respectively) and equal fractions of the bead-bound proteins were analyzed by immunoblotting with α-Kap121p antibodies. Both Nop1p and Kap121p interacted directly with Sof1p.
FIG. 7.
aa 381 to 489 of Sof1p contains a functional Kap121p NLS. (A) GST fusions containing fragments of Sof1p (∼400 ng) or GST alone were immobilized on glutathione-Sepharose 4B beads and incubated with recombinant Kap121p (∼200 ng) or Kap104p (∼300 ng). Equal amounts of the unbound fractions (lanes UB), bound fractions (lanes B), and GST fusions alone (lanes A) were analyzed by SDS-PAGE and Coomassie blue staining. Note that recombinant Kap121p, but not Kap108p, interacts strongly with GST-Sof1p(aa381-498) and weakly with GST-Sof1p(aa411-450). (B) Galactose-inducible GFP fusion proteins containing the four fragments of Sof1p were synthesized at 23°C in kap121-34 cells and analyzed by direct fluorescence microscopy. Note the nucleolar localization of Sof1p(aa381-489)-GFP and the nuclear accumulation of Sof1p(aa411-450)-GFP. (C) The cellular localizations of Sof1p(aa381-489)-GFP and Sof1p(aa411-450)-GFP were determined at both 23 and 37°C in kap121-34 and kap121-34+p_KAP121_ cells. Note that the nuclear import of these Sof1p fragments is dependent on Kap121p function.
FIG. 8.
Heterotrimeric protein complexes containing Sof1p, Kap121p, and Nop1p assemble in vitro. (A) Nop1p-pA was purified from NOP1-A whole-cell lysates, and the copurified proteins were removed by washing them with 1 M MgCl2 (far left panel). The immobilized pA fusion was then incubated with recombinant Kap121p (∼400 ng), forming Nop1p-pA-Kap121p protein complexes (Start). These import complexes were then incubated with transport buffer, GST alone, or GST fusion proteins (∼600 to 800 ng) containing the Kap121p-specific NLS sequences of Nop1p [GST-Nop1p(aa1-90]), Ste12p [GST-Ste12p(aa494-688]), or Pho4p [GST-Pho4p(aa140-166)]. After a washing step, equal volumes of the unbound (lanes UB) and bound (lanes B) fractions were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with α-GST-Kap121p antibodies (which react with GST, protein A, and Kap121p). Note that the addition of the NLS sequences of Nop1p, Ste12p, or Pho4p disrupted the Nop1p-pA-Kap121p import complexes. (B) Sof1p-Nop1p (Sof1p-pA/GST-Nop1p) or Sof1p-Kap121p (Sof1p-pA/Kap121p) complexes were assembled as previously described (Fig. 6D) and incubated with recombinant Kap121p, GST-Nop1p, GST, or transport buffer as indicated. After an extensive washing, the initial complex (lanes B1), unbound (lanes UB), and bound (lanes B2) fractions were prepared for SDS-PAGE, separated, transferred to nitrocellulose, and probed with α-GST-Kap121p antibodies (which react with GST, protein A, and Kap121p). Note that Nop1p dissociates Kap121p from Sof1p, whereas Kap121p interacts with the Sof1p-Nop1p protein complex without displacing Nop1p.
FIG. 9.
Sof1p can be imported by piggyback. (A) Sof1p is imported by interacting with Nop1p bound to a kap. The galactose-inducible SOF1GFP (_GAL1_-Sof1p-GFP) chimera was expressed in kap121-34 cells that contained a plasmid-linked copy of wild-type NOP1 (NOP1), NOP1 fused to a cNLS (NOP1cNLS), galactose-inducible KAP104 (GAL1-KAP104), or galactose-inducible KAP108 (GAL1-KAP108) at permissive (23°C) or restrictive (37°C) temperatures as previously described. Note that the expression of NOP1cNLS or overexpression of KAP104 rescued the cytoplasmic mislocalization of Sof1p-GFP at the nonpermissive temperature (37°C). (C) Whole-cell lysates from the same cultures as those used for microscopy (as shown in panel A) were probed with rabbit polyclonal α-Kap104p, α-Kap108p, α-Gsp1p, and α-GFP and mouse monoclonal α-Nop1p (MAb D77 [5]) antibodies.
Similar articles
- Structural basis for cell-cycle-dependent nuclear import mediated by the karyopherin Kap121p.
Kobayashi J, Matsuura Y. Kobayashi J, et al. J Mol Biol. 2013 Jun 12;425(11):1852-1868. doi: 10.1016/j.jmb.2013.02.035. Epub 2013 Mar 28. J Mol Biol. 2013. PMID: 23541588 - Identification and functional characterization of a novel nuclear localization signal present in the yeast Nab2 poly(A)+ RNA binding protein.
Truant R, Fridell RA, Benson RE, Bogerd H, Cullen BR. Truant R, et al. Mol Cell Biol. 1998 Mar;18(3):1449-58. doi: 10.1128/MCB.18.3.1449. Mol Cell Biol. 1998. PMID: 9488461 Free PMC article. - Karyopherins in nuclear pore biogenesis: a role for Kap121p in the assembly of Nup53p into nuclear pore complexes.
Lusk CP, Makhnevych T, Marelli M, Aitchison JD, Wozniak RW. Lusk CP, et al. J Cell Biol. 2002 Oct 28;159(2):267-78. doi: 10.1083/jcb.200203079. Epub 2002 Oct 28. J Cell Biol. 2002. PMID: 12403813 Free PMC article. - Distinct nuclear import and export pathways mediated by members of the karyopherin beta family.
Moroianu J. Moroianu J. J Cell Biochem. 1998 Aug 1;70(2):231-9. J Cell Biochem. 1998. PMID: 9671229 Review. - Nuclear localization signals for four distinct karyopherin-β nuclear import systems.
Soniat M, Chook YM. Soniat M, et al. Biochem J. 2015 Jun 15;468(3):353-62. doi: 10.1042/BJ20150368. Biochem J. 2015. PMID: 26173234 Review.
Cited by
- Mechanisms and signals for the nuclear import of proteins.
Freitas N, Cunha C. Freitas N, et al. Curr Genomics. 2009 Dec;10(8):550-7. doi: 10.2174/138920209789503941. Curr Genomics. 2009. PMID: 20514217 Free PMC article. - Chaperoning RPA during DNA metabolism.
Li S, Dong Z, Yang S, Feng J, Li Q. Li S, et al. Curr Genet. 2019 Aug;65(4):857-864. doi: 10.1007/s00294-019-00945-3. Epub 2019 Feb 22. Curr Genet. 2019. PMID: 30796471 Review. - Identification of a nuclear localization sequence in β-arrestin-1 and its functional implications.
Hoeppner CZ, Cheng N, Ye RD. Hoeppner CZ, et al. J Biol Chem. 2012 Mar 16;287(12):8932-43. doi: 10.1074/jbc.M111.294058. Epub 2012 Jan 21. J Biol Chem. 2012. PMID: 22267743 Free PMC article. - Kap104p imports the PY-NLS-containing transcription factor Tfg2p into the nucleus.
Süel KE, Chook YM. Süel KE, et al. J Biol Chem. 2009 Jun 5;284(23):15416-24. doi: 10.1074/jbc.M809384200. Epub 2009 Apr 13. J Biol Chem. 2009. PMID: 19366694 Free PMC article. - Artificial nanopores that mimic the transport selectivity of the nuclear pore complex.
Jovanovic-Talisman T, Tetenbaum-Novatt J, McKenney AS, Zilman A, Peters R, Rout MP, Chait BT. Jovanovic-Talisman T, et al. Nature. 2009 Feb 19;457(7232):1023-7. doi: 10.1038/nature07600. Epub 2008 Dec 21. Nature. 2009. PMID: 19098896 Free PMC article.
References
- Aitchison, J. D., G. Blobel, and M. P. Rout. 1996. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science 274:624-627. - PubMed
- Aitchison, J. D., M. P. Rout, M. Marelli, G. Blobel, and R. W. Wozniak. 1995. Two novel related yeast nucleoporins Nup170p and Nup157p: complementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p. J. Cell Biol. 131:1133-1148. - PMC - PubMed
- Allen, N. P., L. Huang, A. Burlingame, and M. Rexach. 2001. Proteomic analysis of nucleoporin interacting proteins. J. Biol. Chem. 276:29268-29274. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases