In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching - PubMed (original) (raw)
In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching
Devin K Binder et al. J Neurosci. 2004.
Abstract
Molecular diffusion in the brain extracellular space (ECS) is an important determinant of neural function. We developed a brain surface photobleaching method to measure the diffusion of fluorescently labeled macromolecules in the ECS of the cerebral cortex. The ECS in mouse brain was labeled by exposure of the intact dura to fluorescein-dextrans (M(r) 4, 70, and 500 kDa). Fluorescein-dextran diffusion, detected by fluorescence recovery after laser-induced cortical photobleaching using confocal optics, was slowed approximately threefold in the brain ECS relative to solution. Cytotoxic brain edema (produced by water intoxication) or seizure activity (produced by convulsants) slowed diffusion by >10-fold and created dead-space microdomains in which free diffusion was prevented. The hindrance to diffusion was greater for the larger fluorescein-dextrans. Interestingly, slowed ECS diffusion preceded electroencephalographic seizure activity. In contrast to the slowed diffusion produced by brain edema and seizure activity, diffusion in the ECS was faster in mice lacking aquaporin-4 (AQP4), an astroglial water channel that facilitates fluid movement between cells and the ECS. Our results establish a minimally invasive method to quantify diffusion in the brain ECS in vivo, revealing stimulus-induced changes in molecular diffusion in the ECS with unprecedented spatial and temporal resolution. The in vivo mouse data provide evidence for: (1) dead-space ECS microdomains after brain swelling; (2) slowed molecular diffusion in the ECS as an early predictor of impending seizure activity; and (3) a novel role for AQP4 as a regulator of brain ECS.
Figures
Figure 1.
_Invivo_loading of brain ECS by fluorescein-dextrans. A, Brain surface exposure after craniectomy showing cortical blood vessels and intact dura. B, Transdural loading of brain ECS showing a cylindrical dam containing aCSF solution of fluorescein-dextran. C, Fluorescence image of cortical surface after dye loading. Scale bar, 1 mm. D, Coronal 300 μm brain slice obtained ex vivo after loading demonstrates fluorescence loading of the cortex. Scale bar, 1 mm. A gradient of fluorescence signal is observed from the cortical surface (top left) to the dorsal hippocampus (bottom right). Arrowhead, Cortical blood vessel; asterisk, white matter.
Figure 2.
Brain ECS diffusion measured by cortical surface photobleaching. A, Schematic of apparatus for cortical surface photobleaching measurements in vivo. B, Photograph showing glass window positioned at the dural surface to dampen cardiorespiratory brain oscillations. C, Representative fluorescence recovery curves for 4 kDa fluorescein-dextran in aCSF (red), brain cortex (black), and aCSF containing 30% glycerol having viscosity ∼2.7 centipoise (blue; spot size, ∼5 μm). D, Left, Relationship between _t_1/2 (half-time) for fluorescence recovery and relative brain versus aCSF diffusion coefficient (D/_D_o; top _x_-axis) deduced from photobleaching measurements on fluorescein-dextran-containing solutions made viscous with glycerol(bottom_x_-axis). See Results for details. Right, Fluorescencere covery curves for a CSF containing 4kDa fluorescein-dextran and indicated concentrations of glycerol.
Figure 3.
Diffusion of fluorescein-dextrans in the brain ECS before and after cytotoxic brain edema. A, Fluorescence recovery curves for indicated fluorescein-dextrans before (black) and at 10 min (blue) and 20 min (green) after intraperitoneal water injection producing cytotoxic brain edema. Both short and long time scales are shown. B, Averaged _t_1/2 (left) and deduced D/_D_o (right) (mean ± SE) from data as in A. The top axis shows equivalent tortuosity (λ = [_D_o/_D_]1/2). C, Percentage of fluorescence recovery (mean ± SE) at 10 sec after photobleaching before and after water intoxication.
Figure 4.
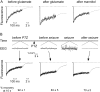
Reduced macromolecular diffusion in the brain ECS after glutamate- and seizure-induced neuronal activity. A, Fluorescence recovery curves for 70 kDa fluorescein-dextran before and 5 min after application of glutamate (1 m
m
) to the cortical surface and 10 min after intraperitoneal injection of mannitol (40 gm/kg). Data are representative of three mice. B, Top, Electroencephalographic recordings before and after intraperitoneal injection of PTZ (100 mg/kg). Bottom, Fluorescence recovery curves for 70 kDa fluorescein-dextran before PTZ administration, after PTZ but before electroencephalographic seizure activity, and after seizure activity. The percentage of fluorescence recovery (mean ± SE; 3 mice) at 10 sec after photobleaching is summarized at the bottom.
Figure 5.
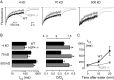
Enhanced ECS diffusion in mice lacking glial water channel AQP4. A, Fluorescence recovery curves for indicated fluorescein-dextrans in the brain cortex of wild-type (black) and AQP4-/- (gray) mice. B, Averaged _t_1/2 (left) and deduced D/_D_o (right) (mean ± SE) from data as in A. The top axis on the right shows equivalent tortuosity (λ = [_D_o/_D_]1/2). *p<0.01 compared with wild type. C, Averaged _t_1/2 (mean ± SE) before and at 10 and 20 min after intraperitoneal water injection.
Similar articles
- Enhanced macromolecular diffusion in brain extracellular space in mouse models of vasogenic edema measured by cortical surface photobleaching.
Papadopoulos MC, Binder DK, Verkman AS. Papadopoulos MC, et al. FASEB J. 2005 Mar;19(3):425-7. doi: 10.1096/fj.04-2834fje. Epub 2004 Dec 13. FASEB J. 2005. PMID: 15596484 - Microfiberoptic fluorescence photobleaching reveals size-dependent macromolecule diffusion in extracellular space deep in brain.
Zador Z, Magzoub M, Jin S, Manley GT, Papadopoulos MC, Verkman AS. Zador Z, et al. FASEB J. 2008 Mar;22(3):870-9. doi: 10.1096/fj.07-9468com. Epub 2007 Oct 26. FASEB J. 2008. PMID: 17965267 - Extracellular space diffusion in central nervous system: anisotropic diffusion measured by elliptical surface photobleaching.
Papadopoulos MC, Kim JK, Verkman AS. Papadopoulos MC, et al. Biophys J. 2005 Nov;89(5):3660-8. doi: 10.1529/biophysj.105.068114. Epub 2005 Sep 2. Biophys J. 2005. PMID: 16143636 Free PMC article. - Three distinct roles of aquaporin-4 in brain function revealed by knockout mice.
Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Verkman AS, et al. Biochim Biophys Acta. 2006 Aug;1758(8):1085-93. doi: 10.1016/j.bbamem.2006.02.018. Epub 2006 Mar 10. Biochim Biophys Acta. 2006. PMID: 16564496 Review. - New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice.
Manley GT, Binder DK, Papadopoulos MC, Verkman AS. Manley GT, et al. Neuroscience. 2004;129(4):983-91. doi: 10.1016/j.neuroscience.2004.06.088. Neuroscience. 2004. PMID: 15561413 Review.
Cited by
- Role of Aquaporins in the Physiological Functions of Mesenchymal Stem Cells.
Zannetti A, Benga G, Brunetti A, Napolitano F, Avallone L, Pelagalli A. Zannetti A, et al. Cells. 2020 Dec 13;9(12):2678. doi: 10.3390/cells9122678. Cells. 2020. PMID: 33322145 Free PMC article. Review. - Astrocyte aquaporin mediates a tonic water efflux maintaining brain homeostasis.
Pham C, Komaki Y, Deàs-Just A, Le Gac B, Mouffle C, Franco C, Chaperon A, Vialou V, Tsurugizawa T, Cauli B, Li D. Pham C, et al. Elife. 2024 Nov 7;13:RP95873. doi: 10.7554/eLife.95873. Elife. 2024. PMID: 39508543 Free PMC article. - Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system.
Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, Deane R, Nedergaard M. Plog BA, et al. J Neurosci. 2015 Jan 14;35(2):518-26. doi: 10.1523/JNEUROSCI.3742-14.2015. J Neurosci. 2015. PMID: 25589747 Free PMC article. - Muddying the water in brain edema?
Smith AJ, Jin BJ, Verkman AS. Smith AJ, et al. Trends Neurosci. 2015 Jun;38(6):331-2. doi: 10.1016/j.tins.2015.04.006. Epub 2015 May 13. Trends Neurosci. 2015. PMID: 25980601 Free PMC article. No abstract available. - Functional hyperemia drives fluid exchange in the paravascular space.
Kedarasetti RT, Turner KL, Echagarruga C, Gluckman BJ, Drew PJ, Costanzo F. Kedarasetti RT, et al. Fluids Barriers CNS. 2020 Aug 20;17(1):52. doi: 10.1186/s12987-020-00214-3. Fluids Barriers CNS. 2020. PMID: 32819402 Free PMC article.
References
- Andrew RD, Fagan M, Ballyk BA, Rosen AS (1989) Seizure susceptibility and the osmotic state. Brain Res 498: 175-180. - PubMed
- Binder DK, Oshio K, Ma T, Verkman AS, Manley GT (2004) Increased seizure threshold in mice lacking aquaporin-4 water channels. NeuroReport 15: 259-262. - PubMed
- Chebabo SR, Hester MA, Aitken PG, Somjen GG (1995) Hypotonic exposure enhances synaptic transmission and triggers spreading depression in rat hippocampal tissue slices. Brain Res 695: 203-216. - PubMed
- Choquet D, Triller A (2003) The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci 4: 251-265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- R01 HL059198/HL/NHLBI NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- HL59198/HL/NHLBI NIH HHS/United States
- Wellcome Trust/United Kingdom
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical