L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus - PubMed (original) (raw)
L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus
Emmanuel G Cormier et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Target cell tropism of enveloped viruses is regulated by interactions between viral and cellular factors during transmission, dissemination, and replication within the host. Binding of viral envelope glycoproteins to specific cell-surface receptors determines susceptibility to viral entry. However, a number of cell-surface molecules bind viral envelope glycoproteins without mediating entry. Instead, they serve as capture receptors that disseminate viral particles to target organs or susceptible cells. We and others recently demonstrated that the C type lectins L-SIGN and DC-SIGN capture hepatitis C virus (HCV) by specific binding to envelope glycoprotein E2. In this study, we use an entry assay to demonstrate that HCV pseudoviruses captured by L-SIGN+ or DC-SIGN+ cells efficiently transinfect adjacent human liver cells. Virus capture and transinfection require internalization of the SIGN-HCV pseudovirus complex. In vivo, L-SIGN is largely expressed on endothelial cells in liver sinusoids, whereas DC-SIGN is expressed on dendritic cells. Capture of circulating HCV particles by these SIGN+ cells may facilitate virus infection of proximal hepatocytes and lymphocyte subpopulations and may be essential for the establishment of persistent infection.
Figures
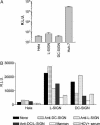
Fig. 1.
L-SIGN- and DC-SIGN-mediated transinfection of HCV pseudoviruses into Huh-7 cells. L-SIGN+, DC-SIGN+, parental HeLa cells, or Huh-7 cells were infected with HCV pseudoviruses. Luciferase activities [relative light units (R.L.U.)] were measured in cell lysates 48 h postinfection. Values are means of three independent experiments ± SD. (A) L-SIGN+, DC-SIGN+, or parental HeLa cells were incubated with HCV pseudovirus-containing supernatants, washed, and cocultured with Huh-7 cells. Luciferase activity in cell lysates was measured 48 h postinfection. (B) Alternatively, DC-SIGN+, L-SIGN+, or parental HeLa cells were preincubated with anti-DC-SIGN mAb 507D (crosshatched bars), anti-L-SIGN mAb 604L (dotted bars), anti-DC/L-SIGN mAb 612X (gray bars), mannan (white bars), HCV+ sera (striped bars), or HCV – sera (data not shown), before addition of pseudoviral supernatants. One representative experiment of three independent experiments is shown.
Fig. 2.
DC-mediated transinfection of HCV pseudoviruses into Huh-7 cells. Immature human DC were differentiated from peripheral blood mononuclear cells of two HCV– donors (A and B) by using granulocyte–macrophage colony-stimulating factor and IL-4. (A) Expression of DC-SIGN was quantified by flow cytometry and represented as mean fluorescent intensity (m.f.i.) of an anti-DC-SIGN mAb. DC were incubated with HCV pseudovirus-containing supernatants, washed, and cocultured with Huh-7 cells. Luciferase activity was measured in cell lysates 48 h postinfection. Direct infection of DC by HCV pseudoviruses was measured in the absence of Huh-7 cells (dotted bars). DC were also preincubated with HCV pseudoviruses alone (black bars) or in the presence of anti-DC/L-SIGN 612X (crosshatched bars), mannan (gray bars), or anti-CD81 mAb JS-81 (white bars). Alternatively, JS-81 was added to DC and Huh-7 cells after pseudovirus capture (dotted bars). (B) Alternatively, DC were incubated with HCV pseudovirus-containing supernatants at 37°C (black bars), 4°C (gray bars), or in the presence of chloroquine (white bars), washed, and cocultured with Huh-7 cells. Luciferase activity was measured in cell lysates 48 h postinfection.
Similar articles
- C-type lectins L-SIGN and DC-SIGN capture and transmit infectious hepatitis C virus pseudotype particles.
Lozach PY, Amara A, Bartosch B, Virelizier JL, Arenzana-Seisdedos F, Cosset FL, Altmeyer R. Lozach PY, et al. J Biol Chem. 2004 Jul 30;279(31):32035-45. doi: 10.1074/jbc.M402296200. Epub 2004 May 27. J Biol Chem. 2004. PMID: 15166245 - Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR.
Pöhlmann S, Zhang J, Baribaud F, Chen Z, Leslie GJ, Lin G, Granelli-Piperno A, Doms RW, Rice CM, McKeating JA. Pöhlmann S, et al. J Virol. 2003 Apr;77(7):4070-80. doi: 10.1128/jvi.77.7.4070-4080.2003. J Virol. 2003. PMID: 12634366 Free PMC article. - Hepatitis C virus targets DC-SIGN and L-SIGN to escape lysosomal degradation.
Ludwig IS, Lekkerkerker AN, Depla E, Bosman F, Musters RJ, Depraetere S, van Kooyk Y, Geijtenbeek TB. Ludwig IS, et al. J Virol. 2004 Aug;78(15):8322-32. doi: 10.1128/JVI.78.15.8322-8332.2004. J Virol. 2004. PMID: 15254204 Free PMC article. - DC-SIGN: binding receptor for HCV?
Feng ZH, Wang QC, Nie QH, Jia ZS, Zhou YX. Feng ZH, et al. World J Gastroenterol. 2004 Apr 1;10(7):925-9. doi: 10.3748/wjg.v10.i7.925. World J Gastroenterol. 2004. PMID: 15052667 Free PMC article. Review. - DC-SIGN: binding receptors for hepatitis C virus.
Wang QC, Feng ZH, Nie QH, Zhou YX. Wang QC, et al. Chin Med J (Engl). 2004 Sep;117(9):1395-400. Chin Med J (Engl). 2004. PMID: 15377434 Review.
Cited by
- Mechanisms of HCV survival in the host.
Sklan EH, Charuworn P, Pang PS, Glenn JS. Sklan EH, et al. Nat Rev Gastroenterol Hepatol. 2009 Apr;6(4):217-27. doi: 10.1038/nrgastro.2009.32. Nat Rev Gastroenterol Hepatol. 2009. PMID: 19347013 Review. - Exploitation of glycosylation in enveloped virus pathobiology.
Watanabe Y, Bowden TA, Wilson IA, Crispin M. Watanabe Y, et al. Biochim Biophys Acta Gen Subj. 2019 Oct;1863(10):1480-1497. doi: 10.1016/j.bbagen.2019.05.012. Epub 2019 May 20. Biochim Biophys Acta Gen Subj. 2019. PMID: 31121217 Free PMC article. Review. - Liver sinusoidal endothelial cells are a site of murine cytomegalovirus latency and reactivation.
Seckert CK, Renzaho A, Tervo HM, Krause C, Deegen P, Kühnapfel B, Reddehase MJ, Grzimek NK. Seckert CK, et al. J Virol. 2009 Sep;83(17):8869-84. doi: 10.1128/JVI.00870-09. Epub 2009 Jun 17. J Virol. 2009. PMID: 19535440 Free PMC article. - DC-SIGN and L-SIGN: the SIGNs for infection.
Khoo US, Chan KY, Chan VS, Lin CL. Khoo US, et al. J Mol Med (Berl). 2008 Aug;86(8):861-74. doi: 10.1007/s00109-008-0350-2. Epub 2008 May 6. J Mol Med (Berl). 2008. PMID: 18458800 Free PMC article. Review. - Liver immunology.
Bogdanos DP, Gao B, Gershwin ME. Bogdanos DP, et al. Compr Physiol. 2013 Apr;3(2):567-98. doi: 10.1002/cphy.c120011. Compr Physiol. 2013. PMID: 23720323 Free PMC article. Review.
References
- Alter, H. J. & Seef, L. B. (2000) Semin. Liver Dis. 20, 17–35. - PubMed
- Lauer, G. M. & Walker, B. D. (2001) N. Engl. J. Med. 345, 41–52. - PubMed
- Cooper, S., Erickson, A. L., Adams, E. J., Kansopon, J., Weiner, A. J., Chien, D. Y., Houghton, M., Parham, P. & Walker, C. M. (1999) Immunity 10, 439–449. - PubMed
- Fry, D. E. & Flint, L. M., Jr. (1997) Bull. Am. Coll. Surg. 82, 8–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R44 AI051134/AI/NIAID NIH HHS/United States
- AI051519/AI/NIAID NIH HHS/United States
- R01 AI060390/AI/NIAID NIH HHS/United States
- P30 AI051519/AI/NIAID NIH HHS/United States
- AI60390/AI/NIAID NIH HHS/United States
- AI051134/AI/NIAID NIH HHS/United States
- R43 AI051134/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases